The sun as a source of energy and catalysts to accelerate chemical reactions were of critical importance in the emergence of the first biochemical molecules on Earth. Yuanxing Fang, Fuzhou University, Fujian, China, Xinchen Wang, Fuzhou University and Fujian Science & Technology Innovation Laboratory for Chemical Engineering of China, Quanzhou, and colleagues have shown that a solid deposited from ammonia and methane plasma can use light energy for amine-to-imine conversions. This process might have played an important role in the creation of the first biomolecules.
Primordial Catalysts
Between three and four billion years ago, on primordial Earth, the first biomolecules were being formed prior to an explosion of life. These early chemical reactions required catalysts. The team discovered that the primordial atmosphere itself could have served as a source for these catalysts.
Using methane and ammonia gases, which were most likely present in the hot gas mixture shrouding the world in the Archean age, the team used chemical vapor deposition to produce nitrogenous carbon compounds, which could serve as catalysts. They found that, in a reaction chamber, molecules condensed out of an ammonia and methane plasma onto a surface quickly grow to form a solid nitrogenous carbon polymer similar to nitrogen-doped graphite.
Imine Formation
The irregularly incorporated nitrogen atoms give the generated polymer catalytically active sites and an electron structure that enables it to be excited by light. The researchers then turned to proving the extent to which the substance can reduce or oxidize other substances under the effect of light.
One of the most significant reactions on early Earth may have been imine formation. Imines, also referred to as Schiff bases, are a dehydrogenated form of amines. Many chemists assume that, on primordial Earth, imines may have been involved in the formation of the first hereditary molecules of ribonucleic acid (RNA). The team showed that their plasma-generated catalyst can convert amines to imines using nothing other than sunlight.
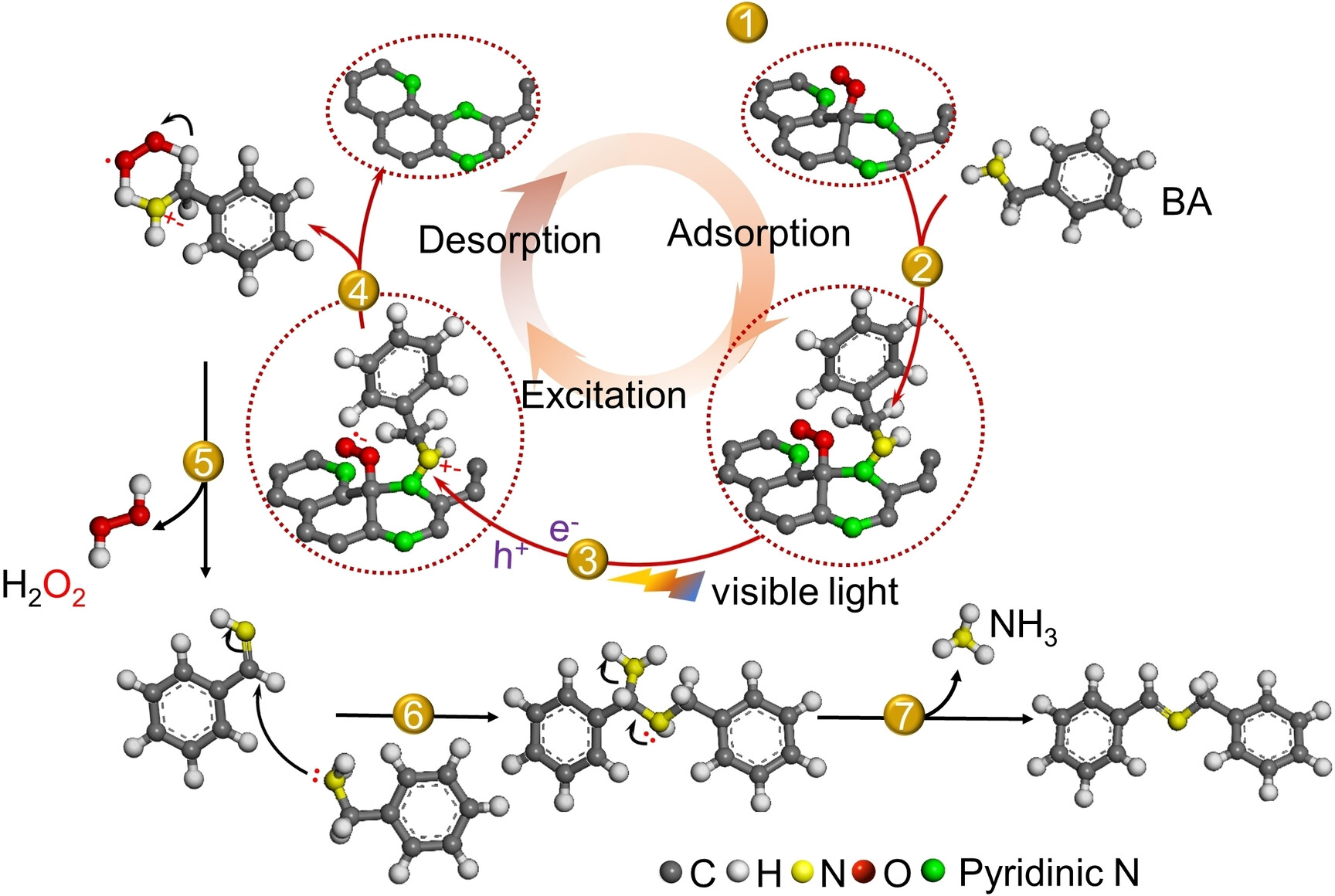
The team performed photoredox reactions using the catalyst in an O2 atmosphere under visible light illumination. A reaction with benzylamine as an example (pictured above) involves the adsorption of O2 and benzylamine on the nitrogen-doped carbon. Under visible light, ⋅O2− is formed and the benzylamine is activated and converted to a cationic radical. One proton from the benzylamine unit is then coupled with ⋅O2− to form H2O2. The generated H2O2 desorbs from the surface. The resulting intermediate is coupled with another benzylamine molecule to form N-benzylidene benzylamine.
The researchers say that carbon-nitride-based photocatalysts such as their plasma-generated substance could have lasted for millions of years and produced important chemical intermediates. In addition, they could also have served as a source of carbon- and nitrogen-containing compounds. By demonstrating that it is possible to produce such a catalyst using only the gases and conditions present in the atmosphere of early Earth, the study sheds new light on the possible formation of early biomolecules.
Reference
- Plasma-Enhanced Chemical-Vapor-Deposition Synthesis of Photoredox-Active Nitrogen-Doped Carbon from NH3 and CH4 Gases,
Yan Wang, Prof. Yuanxing Fang, Yankun Wang, Haisu Wu, Prof. Masakazu Anpo, Jimmy C. Yu, Xinchen Wang,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202307236