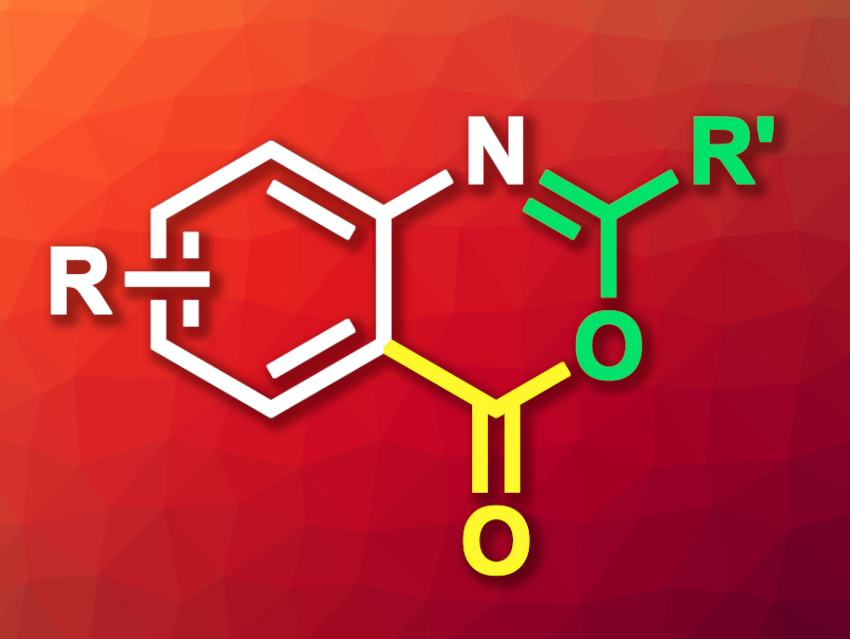
Benzoxazinones are bicyclic heterocycles composed of a benzene and an oxazine ring (a doubly unsaturated six-membered ring containing one oxygen and one nitrogen atom) with a ketone functionality. They can have useful biological activities and act as substrates for the construction of other fused heterocycles. Reacting amide-functionalized arenes with CO in a carbonylation reaction could lead to benzoxazinones when the right catalyst is used.
Yi Lu, Nanjing University, China, and colleagues have developed a Rh(III)-catalyzed direct ortho-C–H bond carbonylation of anilines and their derivatives to construct benzoxazinones. The team either used amide-functionalized arenes directly as the substrates, or employed the corresponding anilines in the presence of excess acetic anhydride to generate acetanilides in situ. They used [Cp*Rh(MeCN)3](SbF6)2 as the catalyst, AgOAc as an oxidant, and dichloroethane (DCE) as the solvent. The reactions were performed at 95 °C.
Under these conditions, a variety of aniline derivatives were reacted with CO (1 atm) to give the desired benzoxazinones. The products were obtained in mostly moderate to excellent yields. For substrates with strongly electron-withdrawing substituents, the reaction gave low yields. From the benzoxazinone products, the corresponding carboxylic-acid-functionalized arenes can easily be obtained by adding water. This can be useful, e.g., for the functionalization of drug-like molecules that contain aniline units.
- Construction of Benzoxazinones from Anilines and Their Derivatives,
Teng-Fei Zhao, Xiao-Li Xu, Wei-Yin Sun, Yi Lu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01511