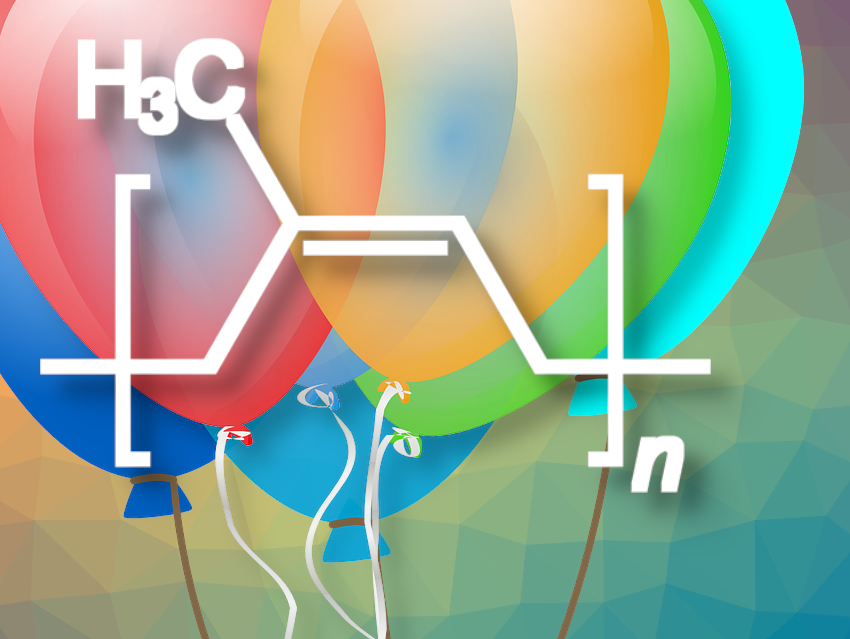Balloons transport us into many a childhood dreamland—and come from the Hevea brasiliensis plant. The transformation of a creamy, white natural substance into a colorful balloon requires a helping hand from the gods, and only Vulcan—the Roman god of fire and a gifted chemist—can perform such a miracle. We have not yet seen through all his tricks, but we will try to follow his chemical trail.
1 Short Historical Outline
Strictly speaking, balloons are a plant product because their skin is made of latex, a milky liquid excreted by some plants when they are damaged. These plants include the dandelion (Taraxacum officinale) commonly seen in Central Europe.
Centuries before the arrival of Columbus, the indigenous peoples of South America produced an elastic and water-repellant material from the “weeping tree” (kau-utshu or cahu-chu). They had observed that latex coagulates when left standing for longer periods of time, forming a thick layer on the surface that could be skimmed off and then dried or smoked. The product was known as rubber (caoutchouc) and the tree as a rubber tree (Hevea brasiliensis). On his second expeditionary voyage to Haiti (1493–1496), Columbus is said to have been the first European to discover the properties of rubber in the form of a toy ball.
The potential of this material was not recognized until two hundred years later, however. French researcher Charles Marie de la Condamine reported in detail on rubber production and its exportation to Europe began in the middle of the 18th century. Rubber has attractive properties: It is malleable, has limited elasticity, and is fully impermeable by water. Its primary disadvantage is temperature sensitivity: It becomes brittle and fragile in the cold and loses its shape and becomes sticky when it is hot.
Despite these disadvantages, it had its uses. English chemist Joseph Priestly used its stickiness in an original way in 1770 when he found it could erase pencil markings. Scottish fabric merchant Charles Macintosh used it to glue together two layers of cloth and used this material to sew waterproof raincoats starting in 1830. These very heavy “macs” reached from the neck to the feet. When it was hot, the wearer became drenched in sweat and the coat became sticky on the outside. Despite this, “mac” remains an English synonym for raincoat to this day.
Today we differentiate between natural and synthetic rubbers, and “rubber” can refer to uncured natural rubber, vulcanized natural rubber, or synthetic rubber.
1.1 The First Big Balloon
In 1783, the Montgolfier brothers flew three hot air balloons (see Fig. 1). The first one had no passengers, the second one held a sheep, a hen, and a duck—and the third, on November 21, carried two French noblemen. The Montgolfier brothers believed that smoke was the source of uplift, so at first, they burned very smoky balls of straw to get going.
 |
|
Figure 1. The first Montgolfière (1783). |
That same year, physics professor Jacques Charles (1746–1823) experimented with balloons that were filled with hydrogen. Filling his balloon dragged on for several days because the hydrogen was generated by pouring 225 kg of sulfuric acid over a half ton of scrap iron. The balloon was surrounded by a net, from which dangled a small boat (see Fig. 2). The silk shell was coated with a solution of rubber in turpentine, which became somewhat gas-tight when dried.
 |
|
Figure 2. La Charlière (1783). |
In his preparatory experiments, Charles discovered the relationship between temperature and volume of an (ideal) gas, which we denote as V = const•T today. The wider public only became aware of this fact through Gay-Lussac after Charles’ death. In contrast to English language textbooks, German texts incorrectly refer to the linear relationship between volume and temperature as the Gay-Lussac law, instead of Charles’ law.
On December 1, 1783, the first gas-filled manned balloon, “La Charlière”, lifted off in the Tuileries (the oldest park in Paris, France). On board were the builder and his colleague Robert. After a short intermediate stop 43 km away, Charles rose to over 2,000 m in altitude in the first solo flight in a balloon. One of the many spectators at this big event was Benjamin Franklin, Ambassador from the recently formed United States of America. When asked what purpose this invention could serve, the 77-year-old replied with a question of his own, “And what is the use of a newborn child?”
Through modern technology, especially improved materials, we can cover large distances in balloons today. In 1999, Bertrand Picard and Brian Jones completed the first non-stop circumnavigation of the earth in just 20 days, and Steve Fosset completed his solo flight around the world in 13 days and 12 hours in 2002.
1.2 The First Small Balloon
The first small toy balloon was made by Michael Faraday in 1824 for his gas experiments at the Royal Institution in London. To make it, he laid one round, thin, rubber sheet on top of another and pressed the edges together to form a gas-tight seal. He dusted the interior with flour to keep the inner surfaces from sticking to each other.
Faraday described his bags as follows: “The caoutchouc is exceedingly elastic. Bags made of it … have been expanded by having air forced into them, until the caoutchouc was quite transparent, and when expanded by hydrogen they were so light as to form balloons with considerable ascending power” [1].
Chemistry enthusiasts today can look back wistfully on the fact that Faraday’s public lectures at the Royal Institution were so popular that the traffic chaos caused by arriving and departing coaches soon caused Albemarle Street to be made the first one-way street in London.
English rubber producer Thomas Hancock seized upon Faraday’s idea and began selling do-it-yourself kits for balloons in 1825. A bubble of a concentrated, viscous solution of rubber in turpentine was blown with a syringe and allowed to dry. This predecessor of our balloons had little in common with our modern version. The balloons were expensive, their surface was unpleasantly sticky, they smelled strongly of turpentine, and they were not very temperature-stable. It took about 150 years for these to evolve into modern balloons, with their beautiful colors, light weight, and durability. Of course, this only happened with the help of chemistry.
2 What Is Rubber?
Michael Faraday determined the elemental composition of rubber to be C5H8 [2] in 1826. In 1860, through careful degradation of rubber, Greville Williams was able to isolate a hydrocarbon of the same composition that boiled at 36 °C, which he called isoprene [3,4]. The structural relationship between liquid isoprene and solid rubber was completely unknown. It was only Hermann Staudiger’s concept of macromolecules, introduced in 1920, that allowed rubber to be seen as a polyisoprene [5].
Staudinger’s stroke of genius was first met with serious opposition. Many leading chemists believed that rubber was a loose agglomeration of many small but well-defined molecules. The fact that Staudinger received the Nobel Prize in 1953, over 30 (!) years after his discovery, illustrates the reluctant acceptance of his peers [6].
 |
|
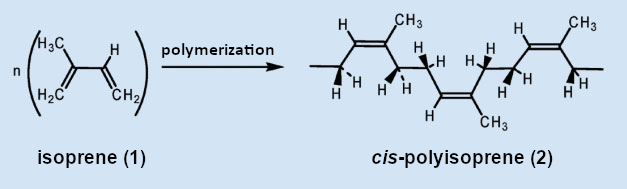
Figure 3. Rubber is a natural polymer from Hevea brasiliensis and consists of isoprene building blocks (1). The double bonds in rubber (2) are in the cis configuration. |
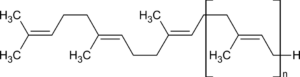
Rubber is a chain polymer of isoprene (2-methyl-1,3-butadiene (1), see Fig. 3), in which the double bonds remaining in the polymer are in the cis configuration. Although the configurations of the double bonds in isoprene are often referred to as cis or trans, these terms cannot, strictly speaking, be used here. Only the Cahn-Ingold-Prelog (CIP) system provides unique stereodescriptors for tri- and tetra-substituted double bonds. According to this system, rubber is in the Z configuration because both higher-ranked substituents on both C atoms of the double bond are on the same side (Z for zusammen, i.e. “together” in German). Some polyisoprene synthesized by other plants (e.g., guttapercha; see Fig. 4) is in the “trans” configuration, which is called the E configuration in the CIP system.
 |
|
Figure 4. Guttapercha. |
References
[1] The History of Balloons, balloonhq.com. (accessed June 5, 2023)
[2] M. Faraday, On the Pure Caoutchouc and the Substances by Which it is Accompanied in the State of Sap or Juice. Quart. J. Sci. 1826, 21, 19–28.
[3] Charles G. H. Williams, On isoprene and caoutchine, Proc. Royal Soc. 1860, 10, 516–519. https://doi.org/10.1098/rspl.1859.0101
[4] A. Gumboldt, Kautschuk nach Maß, Chem. unser Zeit 1967, 1, 41–48. https://doi.org/10.1002/ciuz.19670010203
[5] H. Staudinger, Über Polymerisation, Ber. Dtsch. Chem. Ges. 1920, 53, 1073–1085. https://doi.org/10.1002/cber.19200530627
[6] Herman Staudinger, Macromolecular Chemistry, Nobel Lecture, December 11, 1953.
The article has been published in German as:
- Die Chemie des Luftballons,
Klaus Roth,
Chem. unserer Zeit 2005, 39, 282–289.
https://doi.org/10.1002/ciuz.200590054
and was translated by Caroll Pohl-Ferry.
The Chemistry of Balloons (and Rubber) – Part 2
The discovery of vulcanization—a happy accident or the result of hard work?
The Chemistry of Balloons (and Rubber) – Part 3
How latex is collected, transported, and converted to natural rubber
The Chemistry of Balloons (and Rubber) – Part 4
How balloons are made and why rubber products can contain harmful nitrosamines
See similar articles by Klaus Roth published on ChemistryViews.org