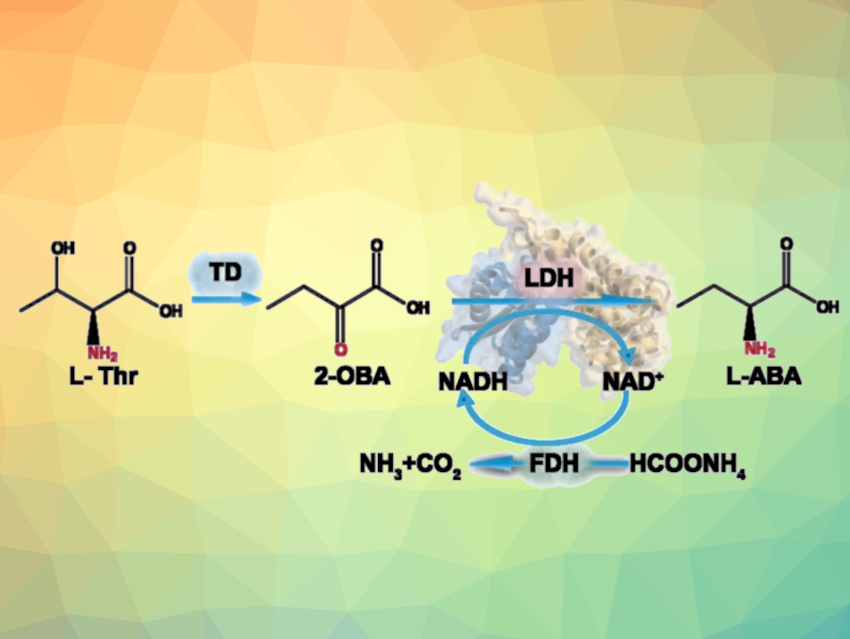
L-2-aminobutyrate (L-ABA, pictured) is an important chiral drug intermediate used to synthesize drugs against, e.g., epilepsy or tuberculosis. For the asymmetric synthesis of L-ABA, biocatalytic methods are particularly interesting. For example, a tri-enzyme cascade pathway using threonine deaminase (TD), leucine dehydrogenase (LDH), and formate dehydrogenase (FDH) can be used to produce L-ABA from L-threonine (pictured, 2-OBA = 2-ketobutyric acid). Optimizing the enzymes used for this could improve their activity and increase yields.
Jing Wu, Jiangnan University, Wuxi, China, and colleagues have developed an efficient method for the asymmetric synthesis of L-ABA using such a tri-enzymatic cascade. The team used the TD enzyme from Corynebacterium glutamicum, the LDH enzyme from Bacillus thuringiensis, and the FDH enzyme from Candida boidinii, which were expressed in E. coli BL21 bacteria. Low activity of the LDH from Bacillus thuringiensis (BtLDH) and an unbalanced expression of enzymes in the cascade were found to be challenges for this approach.
Using mechanism-based protein engineering, the team generated an optimized triple variant of this enzyme, i.e., BtLDHM3(A262S/V296C/P150M) with increased activity and L-ABA yield compared with the wild type. In addition, the team regulated the expression of the three enzymes by optimizing plasmids with different copy numbers per cell, thus optimizing the activity ratio of the enzymes. Using the optimized triple mutant, the researchers achieved a one-pot conversion of L-threonine (L-Thr) to 130.2 gL–1 L-ABA, with 95 % conversion, 99 % e.e. and 10.9 gL–1h–1 productivity (the highest to date, according to the team) in 12 h on a 500 mL scale. Overall, the work describes a potential biosynthesis approach for the industrial production of L-ABA.
- A tri‐enzyme cascade for efficient production of L‐2‐aminobutyrate from L‐threonine,
Xin Li, Changzheng Gao, Wanqing Wei, Wei Song, Weiwei Meng, Jia Liu, Xiulai Chen, Cong Gao, Liang Guo, Liming Liu, Wu Jing,
ChemBioChem 2023.
https://doi.org/10.1002/cbic.202300148