Single-chain nanoparticles (SCNPs) are generated by the folding of individual synthetic polymer chains via crosslinking to form compact structures. SCNPs can be used, e.g., in catalysis. They can, for example, incorporate transition-metal complexes, and thus, combine advantages of heterogeneous and homogeneous catalysis. Photoactivated catalytic SCNPs have also been developed.
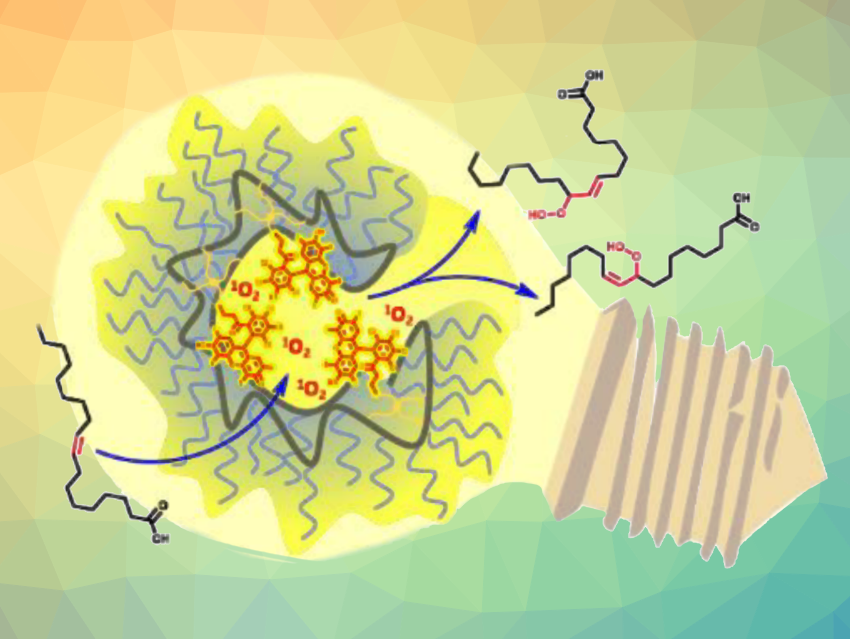
Christopher Barner-Kowollik, Queensland University of Technology, Brisbane, Australia, and colleagues have worked on the construction of SCNP environments that enhance catalysis compared with the corresponding “free”, solution-based process. The team found an SCNP system that can catalyze the photooxidation of nonpolar alkenes up to three times more efficiently than an equivalent small-molecule photosensitizer at the same concentration. They constructed a polymer chain made from poly(ethylene glycol) methyl ether methacrylate and glycidyl methacrylate, which was compacted via multifunctional thiol-epoxide ligation and functionalized with photoactive Rose Bengal.
The resulting SCNP system has a hydrophilic shell and a hydrophobic interior that serves as the catalytic region. It can catalyze the photooxidation of the internal alkene group in oleic acid under green light (pictured). The SCNP’s photocatalytic activity was compared with an identical concentration of free Rose Bengal in solution. The team found that Rose Bengal confined within the SCNP is up to three times more effective for nonpolar alkenes than free RB. They propose that this is due to the proximity of the photosensitizing units to the substrate in the hydrophobic region of the particles.
- Visible Light Reactive Single‐Chain Nanoparticles,
Kai Mundsinger, Bryan T. Tuten, Lily Wang, Kira Neubauer, Christian Kropf, Megan L. O’Mara, Christopher Barner-Kowollik,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202302995