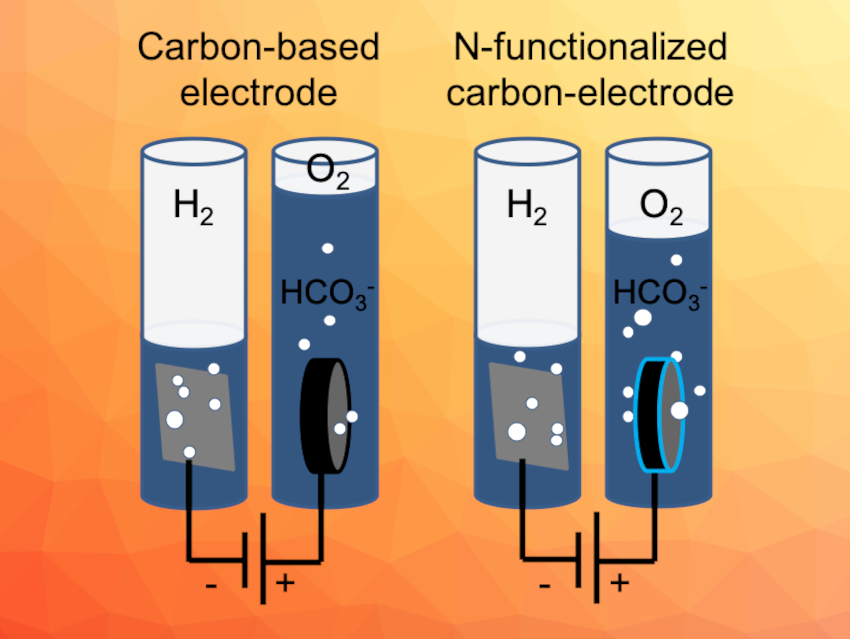
The oxygen evolution reaction (OER) is important for electrochemical water splitting, which could be useful to produce hydrogen and use it as an energy carrier. Suitable electrode materials/catalysts for the OER are interesting research targets. Metal-free carbon materials, for example, are can be synthesized from biomass. Their low price, high conductivity, and the possibility for functionalization make them promising materials in this context.
Saskia Heumann, Anna K. Mechler, Max Planck Institute for Chemical Energy Conversion, Mülheim an der Ruhr, Germany, and colleagues have developed free-standing carbon electrodes produced from glucose via hydrothermal synthesis. The electrodes were prepared either with or without the addition of urotropine as a nitrogen precursor. The resulting electrode materials were investigated as electrodes for the oxygen evolution reaction in alkaline water electrolysis.
The team found that the initially high currents observed for pure carbon electrodes are mostly due to carbon corrosion. This was indicated by a fast degradation of the performance and by CO2 and other carbon species found in the product analysis. In contrast, nitrogen-doped carbon electrodes lasted for more than 300 h in constant operation mode. In this case, significant amounts of oxygen were detected for the first 50 h, showing the material’s catalytic activity.
According to the researchers, the results not only demonstrate the improvement of performance with the addition of nitrogen, but also highlight the importance of detecting the reaction products when working with carbon-based materials, as corrosion processes can have a significant contribution to the observed current.
- Binder‐Free N‐Functionalized Carbon Electrodes for Oxygen Evolution Reaction,
Feihong Song, Jan W. Straten, Yang‐Ming Lin, Yuxiao Ding, Robert Schlögl, Saskia Heumann, Anna K. Mechler,
ChemElectroChem 2023.
https://doi.org/10.1002/celc.202201075