Trifluoromethylsulfones, or triflones, can be useful intermediates in organic synthesis and can have uses, e.g., in pharmaceutical chemistry or functional materials. However, methods for the synthesis of chiral triflones are rare. One possible path for this would be a stereoselective conjugate addition reaction to α-substituted vinyl triflones.
Alena Budinská and Helma Wennemers, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, have developed a mild and efficient organocatalytic method for the stereoselective synthesis of chiral triflones using α-aryl vinyl triflones (pictured below on the left). The reaction gives γ-triflylaldehydes with two non-adjacent stereogenic centers. The team used a bifunctional peptide catalyst with the sequence H-Pro-DMePro-DGln-NH2 (pictured below on the right), which combines a secondary amine and a hydrogen-bond donor, together with i-Pr2NEt. The reactions were performed in CHCl3 at room temperature.
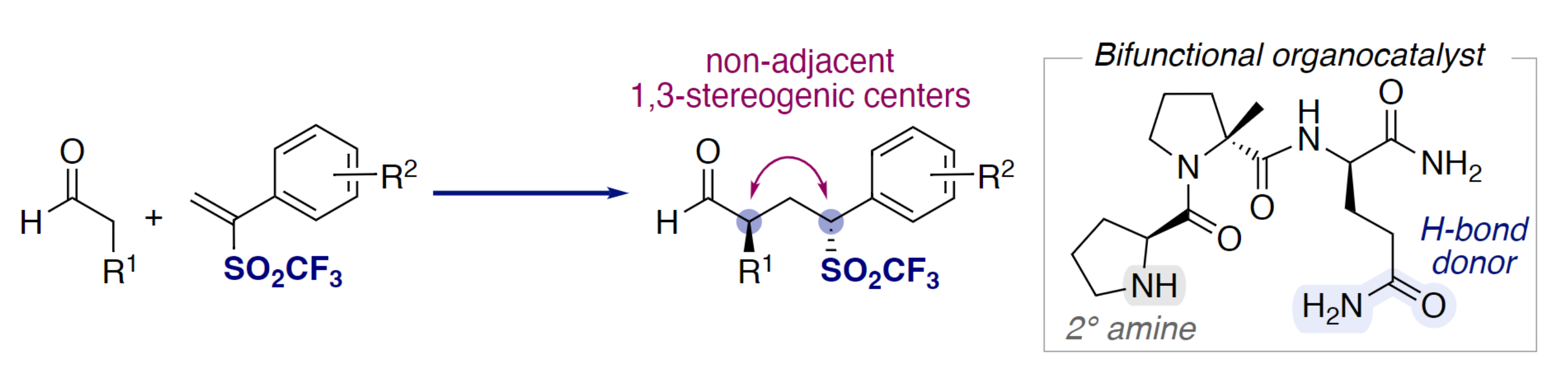
The desired γ-triflylaldehydes were obtained in high yields and stereoselectivities at low catalyst loadings. The team proposes a mechanism that involves a stereoselective conjugate addition–protonation sequence. According to the researchers, the work shows the value of vinyl triflones for the stereoselective synthesis of chiral triflones.
- Organocatalytic Synthesis of Triflones Bearing Two Non‐adjacent Stereogenic Centers,
Alena Budinská, Helma Wennemers,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202300537