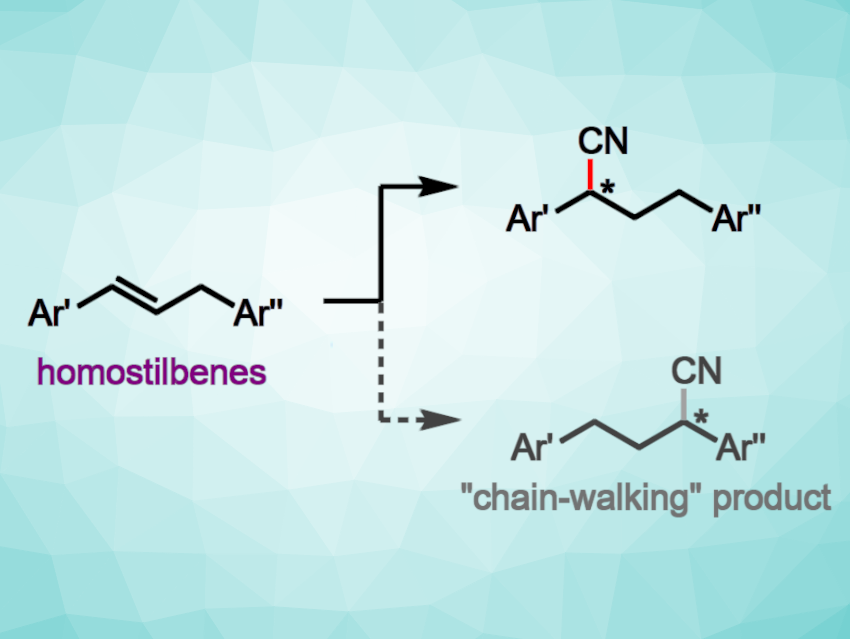
The nickel-catalyzed enantioselective hydrocyanation of 1,3-diarylpropenes (homostilbenes, pictured) represents an interesting selectivity challenge. This is due to a risk of chain-walking by reversible β-H insertion/elimination, which can affect the regioselectivity. Nevertheless, this type of reaction could provide valuable intermediates for the synthesis of bioactive molecules.
Hans-Günther Schmalz and colleagues, University of Cologne, Germany, have investigated enantioselective nickel-catalyzed hydrocyanations using Ni(cod)2 (cod = 1,5-cyclooctadiene) together with a chiral TADDOL-derived phosphite-phosphane ligand and trimethylsilyl cyanide (TMSCN) as a source of HCN. Using this system, the team developed a method for hydrocyanation of various homostilbenes. The required (E)-homostilbene starting materials were prepared in high yields via a palladium-catalyzed coupling of allylic alcohols with aryl-boronic acids.
Despite the risk of chain walking, the hydrocyanation reactions proceeded with useful levels of regio-retention and enantioselectivity (up to 88:12 e.r.) and gave yields of up to 92 %. The team demonstrated the value of the method in a three-step synthesis of a new colchicinoid, i.e., a compound related to the drug colchicine.
- Enantioselective Nickel‐Catalyzed Hydrocyanation of Homostilbenes,
Joss Pepe Strache, Lukas Münzer, Andreas Adler, Dirk Blunk, Hans-Günther Schmalz,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202300050