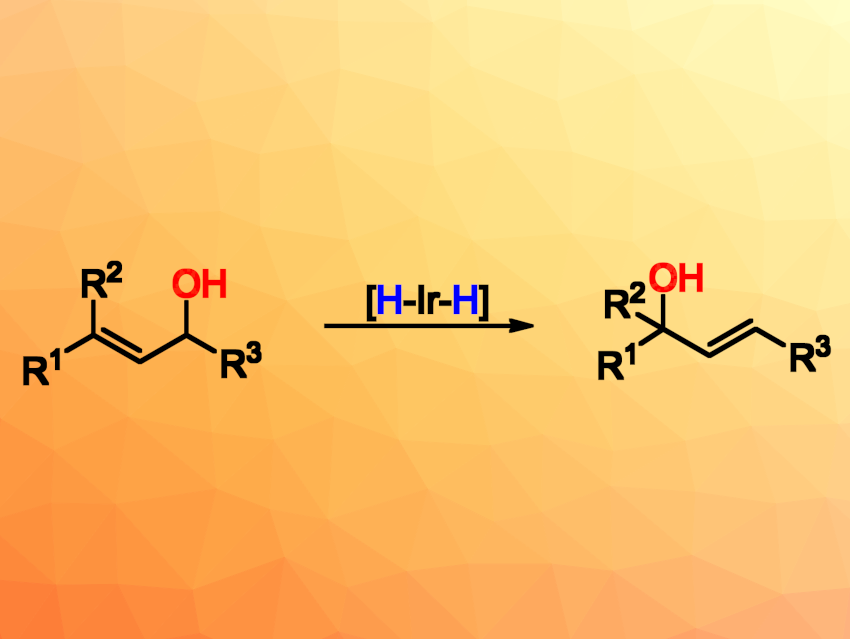
Allylic alcohol units are found in many important molecules and can serve as valuable building blocks in organic synthesis. Therefore, the preparation and transformation of allylic alcohols are interesting research topics. Molecular rearrangements can provide powerful atom- and step-economical synthetic methods. However, research concerning the 1,3-rearrangement of allylic alcohols from readily available substrates to less accessible products is relatively sparse.
Qianjia Yuan, Shanghai Jiao Tong University, China, Wanbin Zhang, Shanghai Jiao Tong University and Zhengzhou University, China, and colleagues have developed a 1,3-rearrangement of allylic alcohols (pictured above) catalyzed by an Ir(III) dihydride complex. The team used Crabtree’s catalyst as a pre-catalyst, which was activated by H2 to form the Ir(III) dihydride complex (pictured below), together with 2-methyltetrahydrofuran (2-MeTHF) as the solvent. The reactions were performed under an N2 atmosphere at room temperature.
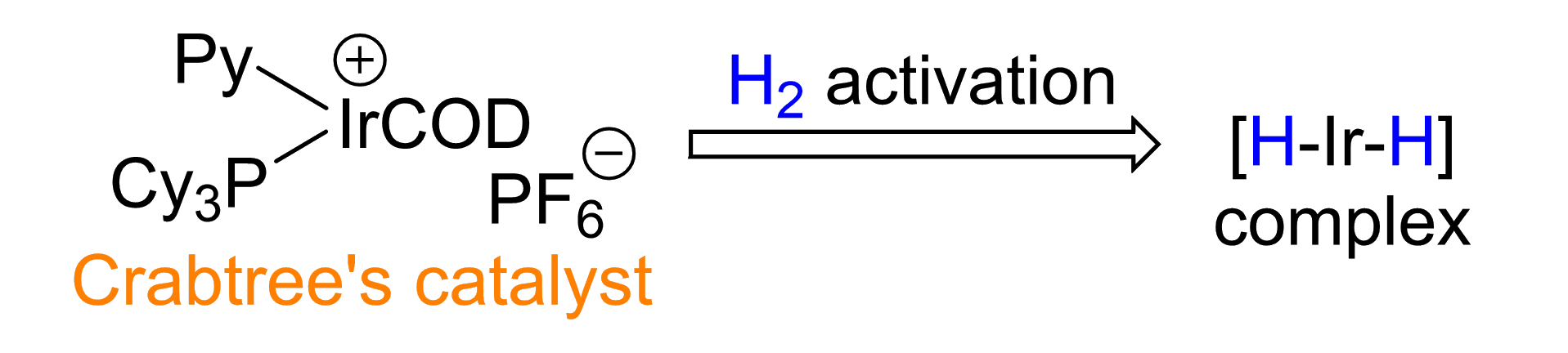
A variety of less accessible allylic alcohols were obtained in good to excellent yields with high regio- and stereoselectivities from readily available E/Z mixtures of the substrates. In addition, the team realized a tandem alkene isomerization/1,3-rearrangement of homoallylic alcohols. They also prepared the natural product navenone B using the developed method. Mechanistic investigations indicate that the reaction pathway involves a π-allyl-Ir(V) intermediate.
- Iridium‐Catalyzed 1,3‐Rearrangement of Allylic Alcohols,
Fei Li, Yicong Luo, Xuejie Zhu, Yong Ye, Qianjia Yuan, Wanbin Zhang,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202300027