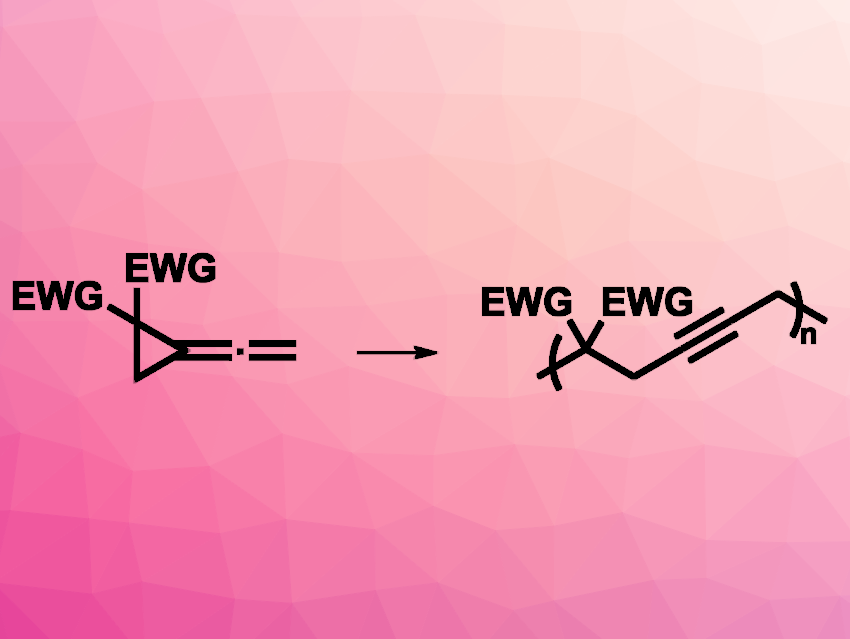
Polymers with alkyne groups in their backbone can have useful properties and options for further functionalization. They can be prepared, e.g., using cumulenes that are prepared in situ. However, cumulenes are unstable, which makes a controlled polymerization difficult. Cyclopropane rings can act as “surrogates” for C═C bonds. Replacing the terminal C═C bond of butatriene, for example, by a cyclopropane ring gives vinylidenecyclopropane (VDCP, derivative pictured above on the left)
Rong Zhu, Peking University, Beijing, China, and colleagues have employed VDCPs as a new monomer class for radical ring-opening polymerization (RROP). The team used a controlled RROP of VDCPs (serving as butatriene homologues) to give poly(VDCP)s (reaction pictured). Azobisisobutyronitrile (AIBN) was used as a radical initiator. The reaction was controlled through reversible addition–fragmentation chain transfer (RAFT) with a chain transfer agent (CTA), using PhCF3 as the solvent.
The team used different VDCP-type monomers, e.g., dimethyl and di(primary alkyl) dicarboxylate derivatives. In addition to the RAFT polymerization, the researchers also performed a photocontrolled polymerization of VDCP under photoinduced-electron-transfer atom-transfer radical polymerization (PET-ATRP) conditions. For this variant, they used an iridium-based photocatalyst and an alkyl bromide initiator under blue light irradiation. Overall, poly(VDCP)s were prepared with high structural regularity and good control over the molecular weights.
- Alkyne Polymers from Stable Butatriene Homologues: Controlled Radical Polymerization of Vinylidenecyclopropanes,
Bin Wu, Qian-Jun Ding, Zheng-Lin Wang, Rong Zhu,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.2c12220