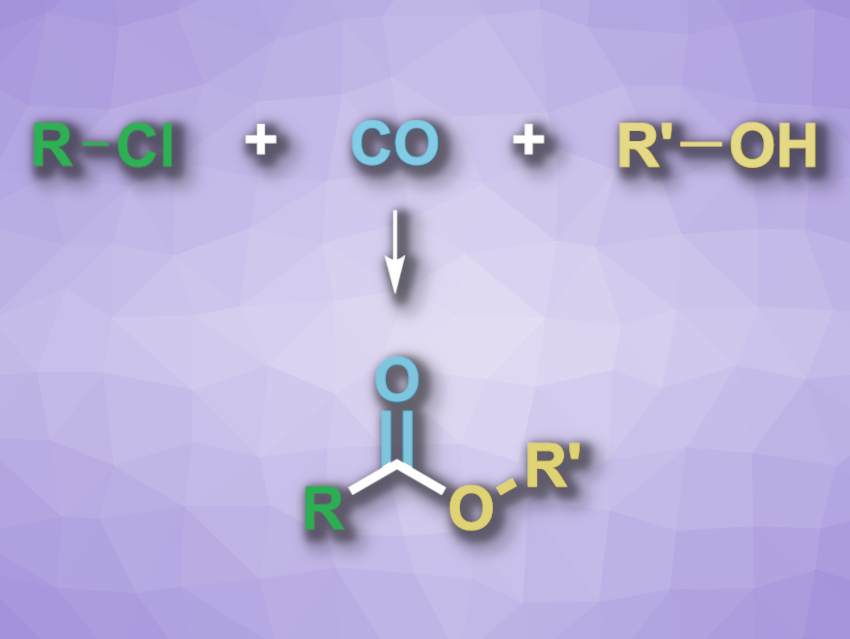
Alkyl chlorides often are cheap feedstocks that can be useful for the synthesis of organic compounds. However, the C(sp3)–Cl bond can be difficult to activate. For example, while the catalytic alkoxycarbonylation of alkyl halides can be used to synthesize esters, this type of reaction is often limited to iodides and bromides. Alkyl chlorides as alternative substrates could be cheaper and less toxic.
Xiao-Feng Wu, Leibniz-Institut für Katalyse e.V., Rostock, Germany, Dalian National Laboratory for Clean Energy and Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and colleagues have developed an approach to the manganese-catalyzed alkoxycarbonylation of unactivated alkyl chlorides (reaction pictured), which can be used to prepare esters. The team used a manganese PNP-type pincer complex as the catalyst, Cs2CO3 as a base, and cumene as the solvent. The reactions were performed at 140 °C under 6 bar CO.
Under these conditions, a variety of alcohols and alkyl chlorides were reacted to prepare the corresponding esters. The desired products were obtained in moderate to high yields, depending on the substrates. The team proposes a reaction mechanism that involves an oxidative addition between the active Mn-based catalyst and the alkyl chloride, followed by a migratory insertion of CO and a nucleophilic attack of the alcohol to form the desired ester. A reductive elimination then regenerates the catalyst.
- Manganese-Catalyzed Alkoxycarbonylation of Alkyl Chlorides,
Han-Jun Ai, Hui-Qing Geng, Xing-Wei Gu, Xiao-Feng Wu,
ACS Catal. 2023.
https://doi.org/10.1021/acscatal.2c05854