The use of alkaline earth metals in catalysis is an emerging research field. This approach could be useful due to the high abundance of these metals in the Earth’s crust, which makes them valuable alternatives to the transition metals that are traditionally used in catalytic transformations. Magnesium is a particularly interesting candidate, as it exhibits high biocompatibility and low toxicity.
From an environmental point of view, a high atom economy is desirable for any reaction with regards to sustainability and the avoidance of waste. In general, hydroelementation and dehydrocoupling reactions are highly atom-economic processes, providing access to a variety of compounds,
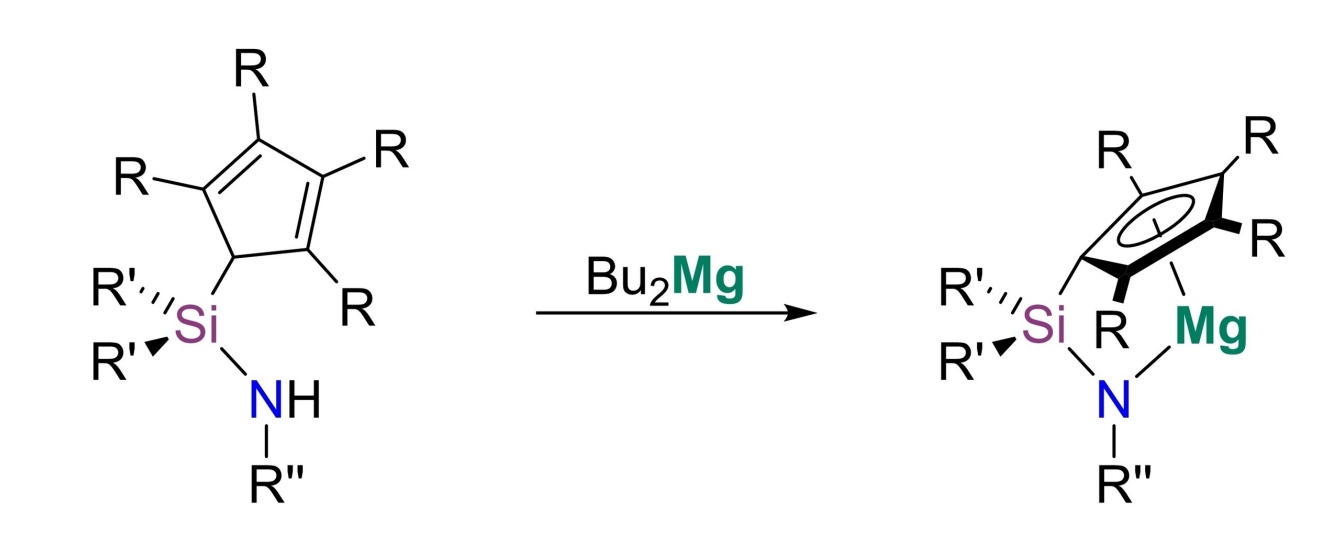
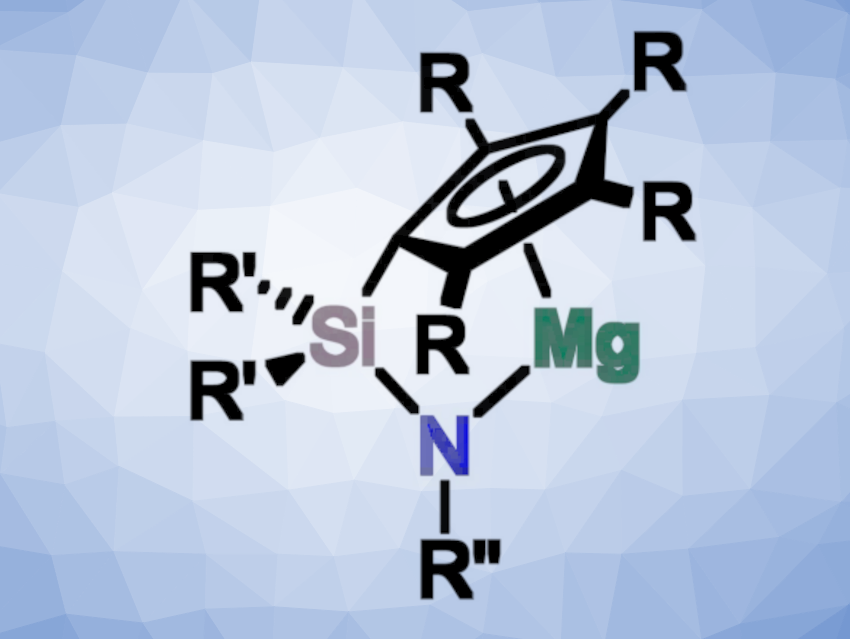
Bringing together these two aspects, André Schäfer and colleagues, Saarland University, Saarbrücken, Germany, have developed a series of structurally constrained magnesium complexes, which can act as potent catalysts in hydroelementation and dehydrocoupling reactions. The team prepared four different ansa-half-sandwich complexes of magnesium (pictured, R = H, Me; R’ = Me, Ph; R” = tBu, Ph) via reactions of the corresponding ligands with dibutylmagnesium.
Using these catalysts, the dehydrocoupling of different amine-boranes and the cross-dehydrocoupling of amines and silanes is possible at ambient conditions and with low catalyst loadings. The team also showed that the new catalysts can catalyze the hydroboration of a variety of substrates such as alkynes, nitriles, and imines, as well as the hydroacetylation of carbodiimides and the intramolecular ring-closing hydroamination of an α,ω-aminoalkene. While other s-block-based catalysts for these reactions are known, such versatile catalysts are quite rare. The system, thus, represents an interesting advancement in the field of homogeneous magnesium catalysis.
- Constrained Geometry ansa‐Half‐Sandwich Complexes of Magnesium – Versatile s‐Block Catalysts,
Lisa Wirtz, Kinza Yasmin Ghulam, Bernd Morgenstern, André Schäfer,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202201007

![Unique Features of the Dinuclear Zirconocene Complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)]](https://www.chemistryviews.org/wp-content/uploads/2025/03/202503_Dinuclear-Zirconocene-Complex-125x94.png)