Bioorthogonal chemistry refers to chemical reactions that can occur inside living systems without interfering with native biochemical processes. For example, the [4+2] cycloaddition of 1,2,4,5-tetrazines and trans-cyclooctenes shows good reaction kinetics, high selectivity, and compatibility with biological systems. These properties enable its use in living organisms.
Matthias Manfred Herth, University of Copenhagen, and Rigshospitalet, Copenhagen, Denmark, and colleagues have synthesized a tetrazine compound and radiolabeled it using tritium (3H). According to the researchers, this is the first 3H-labeled tetrazine. Tritium is a radioactive isotope of hydrogen. It is commonly used in medicinal chemistry as a label to, e.g., follow the course of a drug in the human body.
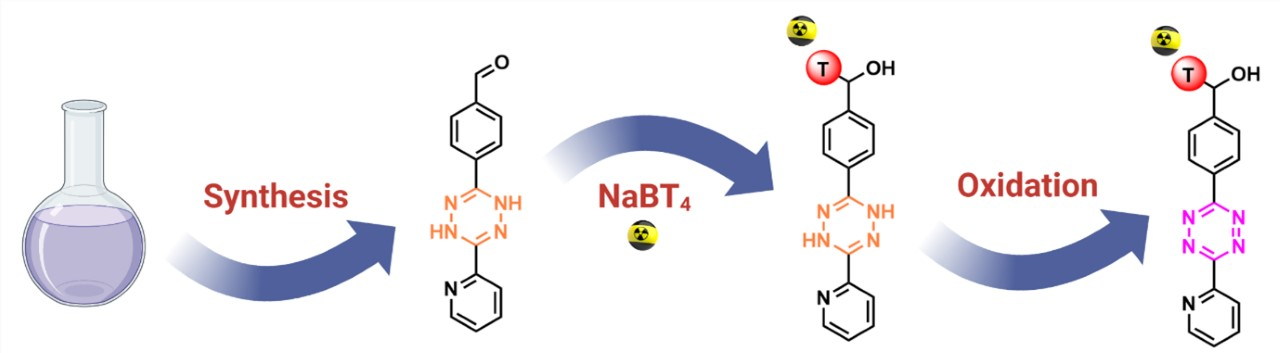
The tritiated tetrazine compound was prepared from the corresponding aldehyde via a simple reduction using NaBT4 (pictured below). The labeled tetrazine was obtained with a radiochemical yield of 22 % and a radiochemical purity of 96 %.
The tritiated tetrazine was then used to radiolabel a trans-cyclooctene-modified antibody at room temperature—without any need for purification. The antibody completely retained its affinity and selectivity in an in vitro autoradiography experiment. Autoradiography is an imaging technique that uses radioactive sources contained directly within the sample. This work might be helpful for the development of other tritiated tetrazines with possible applications for in vitro or ex vivo assays.
- Development of the First Tritiated Tetrazine: Facilitating Tritiation of Proteins,
Natasha Shalina Radjani Bidesi, Umberto Maria Battisti, Sara Lopes van de Broek, Vladimir Shalgunov, Anne‐Mette Dall, Jesper Bøggild Kristensen, Dag Sehlin, Stina Syvänen, Gitte Moos Knudsen, Matthias Manfred Herth,
ChemBioChem 2022.
https://doi.org/10.1002/cbic.202200539