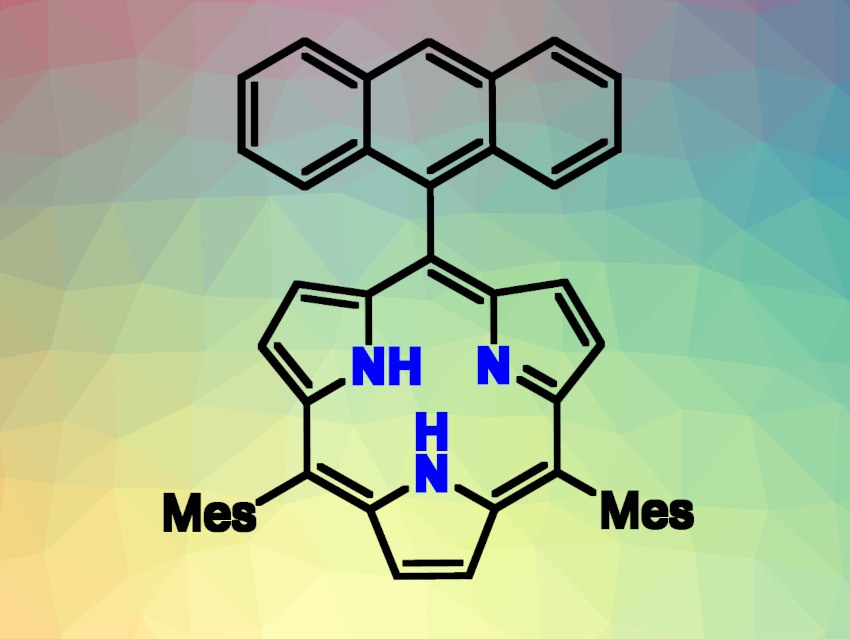
Subporphyrins are ring-contracted porphyrins that consist of three pyrroles and three meso-carbons. B(III) subporphyrins, which feature a boron center, are known species with curved, bowl-like structures, 14π-aromaticity, and unique photophysical properties. However, subporphyrin free bases (example pictured) had remained elusive so far, despite attempts to remove the boron from B(III) subporphyrins.
Dongho Kim, Yonsei University, Seoul, Republic of Korea, Pohang University of Science and Technology (POSTECH), Republic of Korea, Atsuhiro Osuka, Jianxin Song, Hunan Normal University, Changsha, China, and colleagues have synthesized subporphyrin free bases. The team initially attempted the coupling of an α,α’-diborylated tripyrrane with 1,1-diphenyl-2,2-dibromoethene, followed by an oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). They obtained a complicated mixture, from which a subporphyrinoid without 14π-aromaticity was isolated.
The researchers then incorporated an anthracene precursor unit into the brominated coupling partner. The recovery of the aromaticity of anthracene helps to promote the desired reaction. This coupling reaction gave the subporphyrin free bases, one with and one without a COOH group (latter pictured), as well as a free base dimer.
Similar to their B(III) complexes, the subporphyrin free bases exhibit curved bowl-like structures and distinct 14π-aromaticity. The excited-state behaviors of the subporphyrin free bases are also comparable with those of the corresponding B(III) subporphyrins. This work might open a path to the development of new subporphyrin complexes.
- Synthesis of Subporphyrin Free Base,
Le Liu, Jinseok Kim, Ling Xu, Yutao Rao, Mingbo Zhou, Bangshao Yin, Juwon Oh, Dongho Kim, Atsuhiro Osuka, Jianxin Song,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202214342