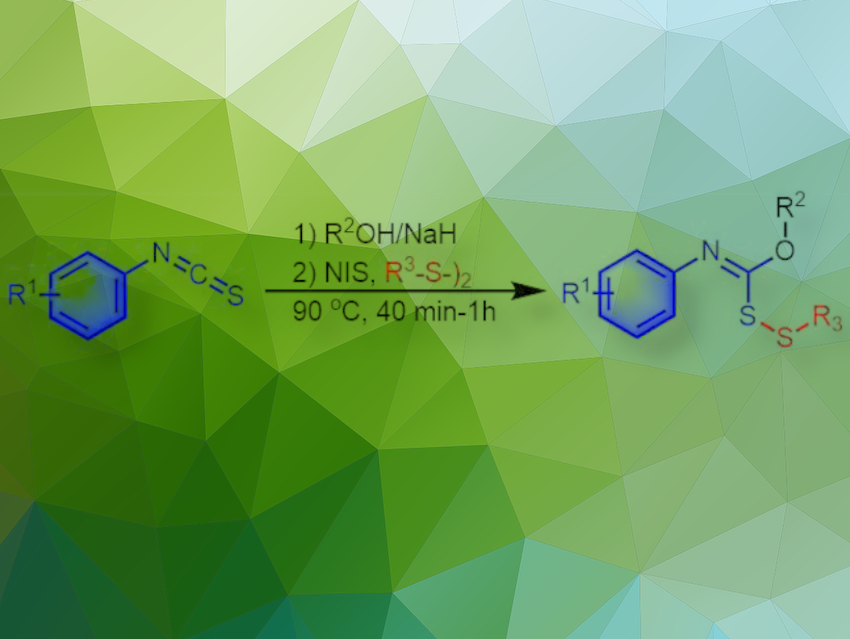
Disulfides are indispensable compounds in organic chemistry. They play important roles in biochemistry, pharmaceutical chemistry, materials chemistry, life sciences, and synthetic chemistry because of their pharmaceutical properties and biological activities. Therefore, the synthesis of disulfides has attracted the interest of many chemists. Compared to symmetrical disulfides, the synthesis of unsymmetrical disulfides is still relatively undeveloped. Some of the reported methods use transition metal catalysts, foul-smelling reagents, and pre-functionalized starting materials. Thus, it is desirable to develop more efficient S-S bond preparation strategies to obtain unsymmetrical disulfides.
Zhi-Bing Dong, Wuhan Institute of Technology, China, and colleagues have reported an efficient and convenient method for the synthesis of unsymmetrical disulfides. The group used isothiocyanates as substrates and N-iodosuccinimide (NIS) as a promoter to obtain various unsymmetrical disulfides in moderate to good yields under transition-metal-free conditions. This method is characterized by mild conditions, easy handling, short reaction time, and a wide range of substrates.
Mechanistically, the control experiments showed that the addition of radical trapping agents such as TEMPO (2,2,6,6-tetramethyl-1-piperinedinyloxy) and BHT (butylated hydroxytoluene) would lead to a significant erosion of the product. Therefore, the researchers suspect that the reaction might proceed via a radical pathway.
Remarkably, the desired products could be obtained in moderate to good yields from inexpensive and easily available starting materials, providing an alternative way of producing various unsymmetrical disulfides. According to the researchers, this protocol extends their previous research in organosulfur chemistry based on green synthesis.
- N-Iodosuccinimide (NIS) Promoted Synthesis of Unsymmetrical Disulfides Starting from Isothiocyanates Under Transition-Metal-Free Conditions,
Cheng-Li Yang, Hua-Qing Xiao, Zhi-Bing Dong,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200954