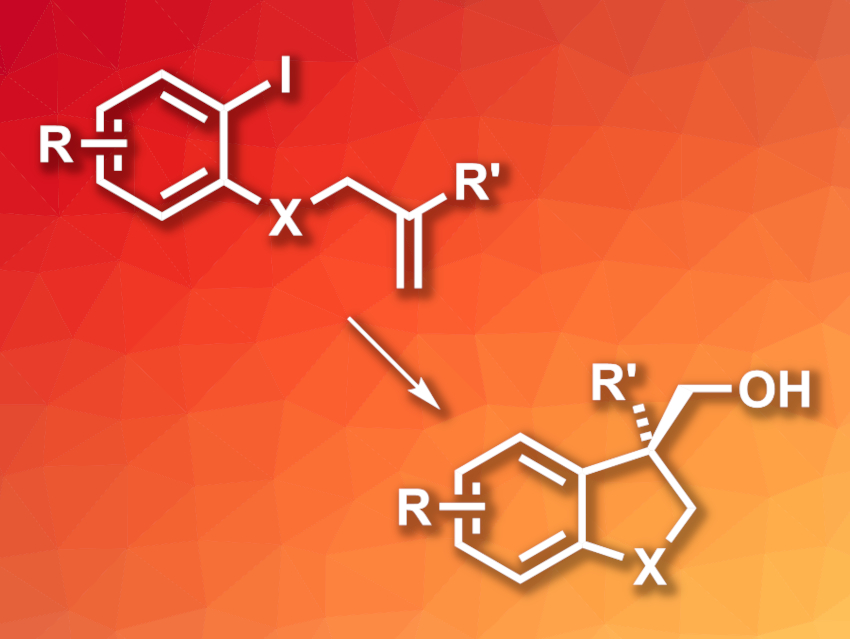
Nitrous oxide (N2O) is a potent greenhouse gas, but can also be useful, e.g., in medicine or as an oxygen transfer reagent in chemistry. When used as an oxygen transfer reagent, it releases non-toxic nitrogen. However, it can be challenging to activate N2O using transition-metal catalysts for use in organic synthesis.
Josep Cornella, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed a protocol for the catalytic synthesis of cycloalkanols (pictured, X = O, CH2), using N2O as an oxygen transfer agent. The team used NiI2 as a catalyst together with a phenanthroline-based ligand to convert alkene-substituted aryl iodides to the corresponding cycloalkanols in the presence of NaI and activated zinc. The reactions were performed in dimethyl sulfoxide (DMSO) as the solvent under 2.5 atm of N2O at 25 °C.
The desired products were obtained in generally moderate to high yields. The researchers also tested the same protocol with a chiral, imidazolylpyridine-based ligand. They found that the reaction can be performed in an enantioselective manner with high enantioselectivities when such chiral ligands are used. Mechanistic studies indicate that N2O is the oxygen transfer reagent in this protocol. This could also be useful for the synthesis of other oxygenated compounds in organic synthesis.
- Ni-Catalyzed Oxygen Transfer from N2O onto sp3-Hybridized Carbons,
Shengyang Ni, Franck Le Vaillant, Ana Mateos-Calbet, Ruben Martin, Josep Cornella,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c06227