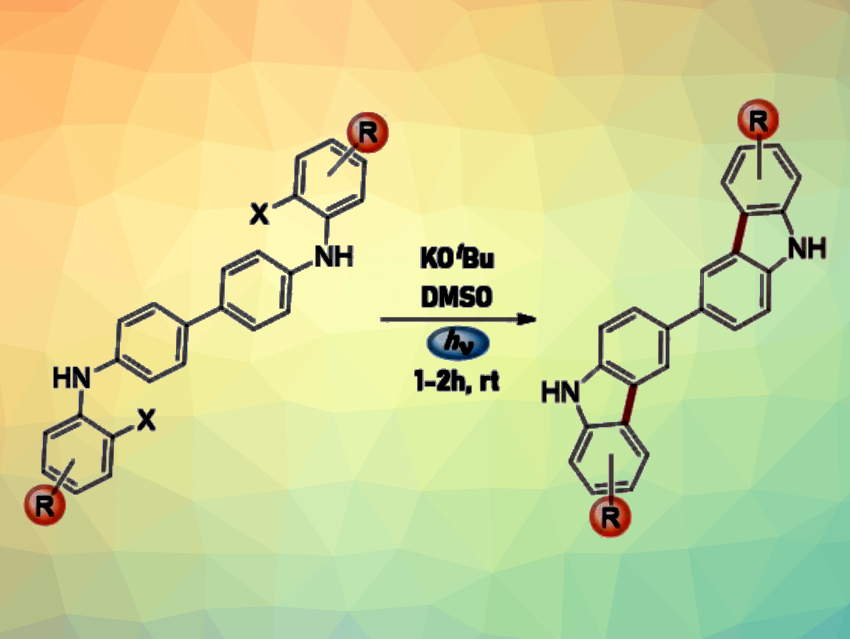
Bicarbazoles and indolocarbazoles are nitrogen-containing heterocycles. They can be used in emitting materials for organic light-emitting diode (OLED) technology. The synthesis of these compounds usually requires the use of transition metals, high temperatures, acids, and/or oxidants. Light is a powerful tool in synthesis that does not generate waste products and simplifies purification processes. Thus, methods for organic syntheses involving light have garnered research interest.
María Eugenia Budén, Instituto de Investigaciones en Físicoquímica de Córdoba (INFIQC), Córdoba, Argentina, and colleagues have developed a method for the synthesis of 3,3′-bicarbazoles (general reaction pictured) and indolocarbazoles from diarylamines in the presence of a base and visible light at room temperature. The team used a variety of N4,N4′-bis(2-halophenyl)-[1,1′-biphenyl]-4,4′-diamines and N,N-bis(2-halophenyl)benzene-diamines as substrates for the synthesis of 3,3′-bicarbazole and indolocarbazole derivatives, respectively. Potassium tert-butoxide (KOtBu) was used as a base and dimethyl sulfoxide (DMSO) as the solvent. The reactions were performed under blue LED light.
The team proposes that the reaction proceeds via an intramolecular, photoinduced SRN1 mechanism. The addition of KOtBu solution leads to the formation of the corresponding dianions. Exposure to light probably leads to the formation of excited dianions, which could be the species responsible for triggering the reaction.
The desired products were obtained in moderate to good yields. The reaction tolerates a variety of functional groups. Overall, the developed protocol could be useful for the preparation of photoluminescent compounds.
- Construction of 3,3’‐Bicarbazoles and Indolocarbazones by Using Visible Light,
María Alexia El Ain, Marcelo Puiatti, María Eugenia Budén,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200642