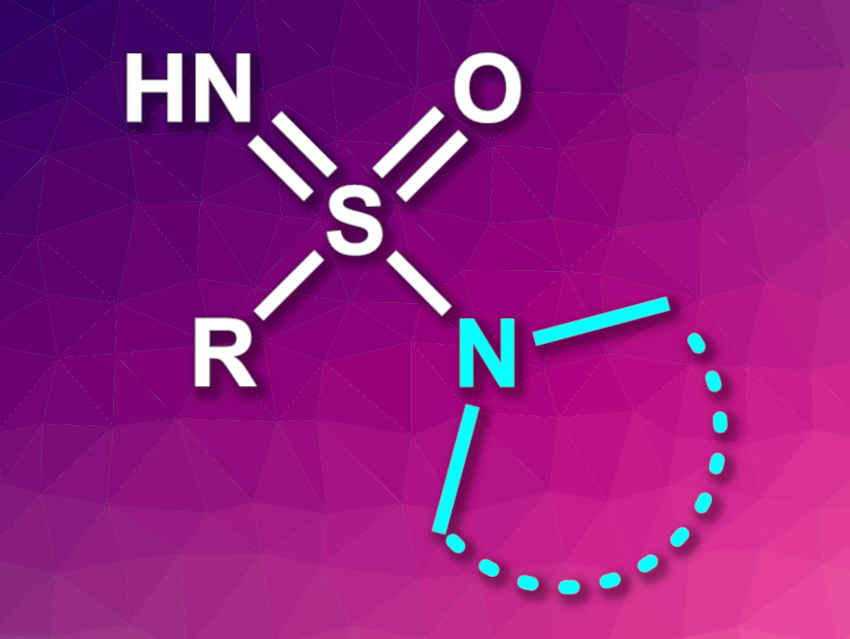
Sulfonamides are widely used in pharmaceutical chemistry and agrochemistry. Sulfonimidamides are aza-analogues of sulfonamides and can also be bioactive. NH-unsubstituted sulfonimidamides are particularly interesting because they can take part in hydrogen bonds and are more hydrophilic than N-functionalized derivatives. Existing synthesis methods often lead to N-functionalized sulfonimidamides, requiring an additional step to obtain unsubstituted derivatives. Alternative approaches can need, for example, highly reactive oxidants.
Carsten Bolm, RWTH Aachen University, Germany, and colleagues have developed a method for the direct synthesis of NH-sulfonimidamides via the copper-catalyzed coupling of primary sulfinamides with secondary amines, using air as the terminal oxidant. The team used a broad range of primary sulfinamides and both cyclic and acyclic amines as substrates. They were reacted using CuBr2 as the catalyst and toluene as the solvent under air at room temperature.
The desired sulfonimidamides were obtained in generally good yields. The team proposes a mechanism that involves a copper-bound sulfanenitrile as a key intermediate. The reaction shows a good functional group tolerance, uses air as the only oxidant, and requires no pre-functionalized reactants or additives.
- Copper-Catalyzed, Aerobic Synthesis of NH-Sulfonimidamides from Primary Sulfinamides and Secondary Amines,
Peng Wu, Joachim Demaerel, Deshen Kong, Ding Ma, Carsten Bolm,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c02804