Industrialization has led a fossil-fuel-dependent economy. However, these fuels are finite, and greener and renewable replacements are needed. Biofuels can be a part of the answer and can also reduce CO2 emissions. Biodiesel production yields approximately 10 % glycerol as a by-product. Economical ways to use this byproduct would, thus, be useful. Propylene, for example, is a useful resource and usually also generated from fossil feedstocks. Excess glycerol could be converted to propanol, which, in turn, could serve as an intermediate in the sustainable production of propylene.
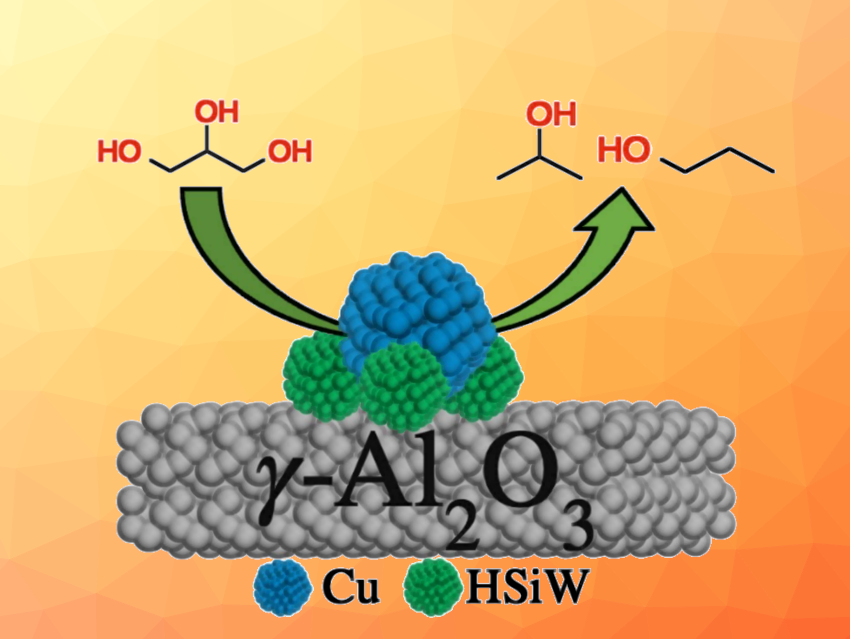
Holger B. Friedrich, University of KwaZulu-Natal, Durban, South Africa, and colleagues have explored the valorization of glycerol to form bio-propanols and other valuable chemicals. The team studied bifunctional copper–tungstosilicic acid (HSiW) catalysts, supported on γ-Al2O3, for the continuous flow hydrogenolysis of glycerol. They prepared three catalysts with varying amounts of HSiW.
The best-performing catalyst, with 26 wt% Cu and 14 wt% HSiW, achieved a 100 % conversion of glycerol after 2 h and 51 % selectivity to propanols (27 % 1-propanol and 24 % 2-propanol).
According to the researchers, the HSiW introduces porosity and acidity that improve the performance of the bifunctional catalysts. This study offers insights into the nature of the active sites required for the hydrogenolysis reaction and showed it can be achieved without using expensive platinum-group metals. In addition, performing this reaction in a continuous flow reactor has considerable industrial advantages compared to a batch process.
- Sustainable selective propanol production via continuous flow conversion of glycerol over synergistic bifunctional catalysts: An exploration into factors affecting activity.,
Aaron L. Folkard, Majid D. Farahani, Abdul S. Mahomed, Holger Bernhard Friedrich,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200602