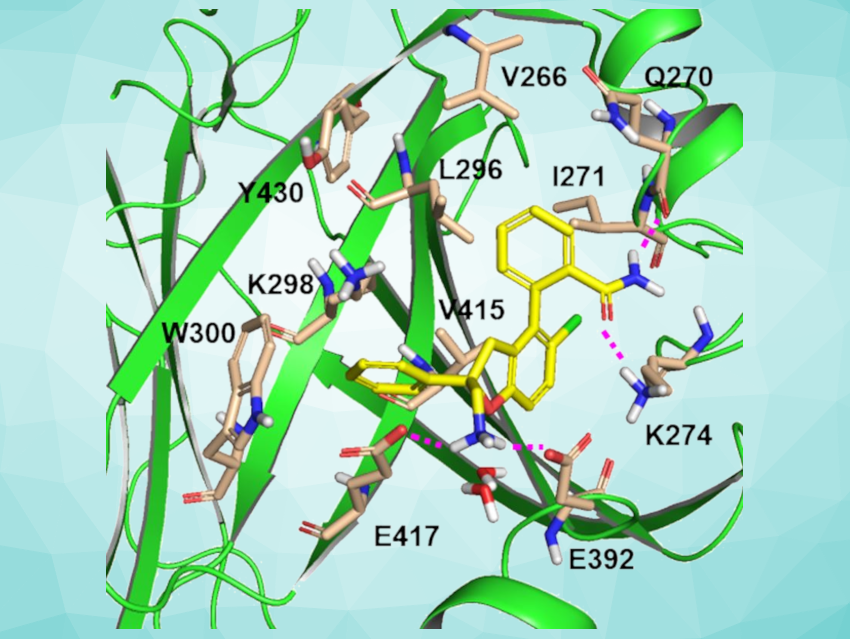
The protein–protein interaction between two proteins called YAP and TEAD is an important therapeutic target in anticancer drug research. The two proteins are involved in a signaling pathway that plays a role in regulating cell proliferation and apoptosis (programmed cell death). However, because the surface of interaction of the two proteins is comparatively large, identifying small molecules that can inhibit the binding of YAP to TEAD is a challenge in medicinal chemistry.
Pascal Furet, Novartis Institutes for Biomedical Research, Basel, Switzerland, and colleagues have discovered the first class of small molecules that can potently disrupt the YAP–TEAD interaction. Using crystal structure information, the team performed a virtual screening of the Novartis compound collection. Promising hits were assessed biochemically using an assay that measures their ability to inhibit the interaction between peptides corresponding to parts of YAP and TEAD. Their protein binding properties were also tested using NMR spectroscopy.
This approach led the team to a weakly active inhibitor of the YAP–TEAD interaction. Structure-based design was then used to synthesize promising analogues of this inhibitor. The team was able to improve the potency by five orders of magnitude. The resulting compounds (example pictured in a complex with TEAD) can block the activity of TEAD, and they show a robust antiproliferative effect in cellular assays. They could provide a path for the development of clinical candidates to treat cancers associated with a dysregulation of the targeted signaling pathway.
- The First Class of Small Molecules Potently Disrupting the YAP‐TEAD Interaction by Direct Competition,
Pascal Furet, Vincent Bordas, Mickaël Le Douget, Bahaa Salem, Yannick Mesrouze, Patricia Imbach-Weese, Holger Sellner, Markus Voegtle, Nicolas Soldermann, Emilie Chapeau, Markus Wartmann, Clemens Scheufler, Cesar Fernandez, Joerg Kallen, Vito Guagnano, Patrick Chène, Tobias Schmelzle,
ChemMedChem 2022.
https://doi.org/10.1002/cmdc.202200303