Silk has been used in sutures for centuries and continues to be a versatile material for medical use. Due to its excellent biocompatibility and strength-to-weight profile, silk fibroin, a protein from the silkworm cocoon, has applications, e.g., in stents, arterial grafts, heart valves, repair meshes, and more. However, when used in living organisms, such materials can suffer from fouling, act as sites of infection, or suffer from loss of function due to water uptake and swelling.
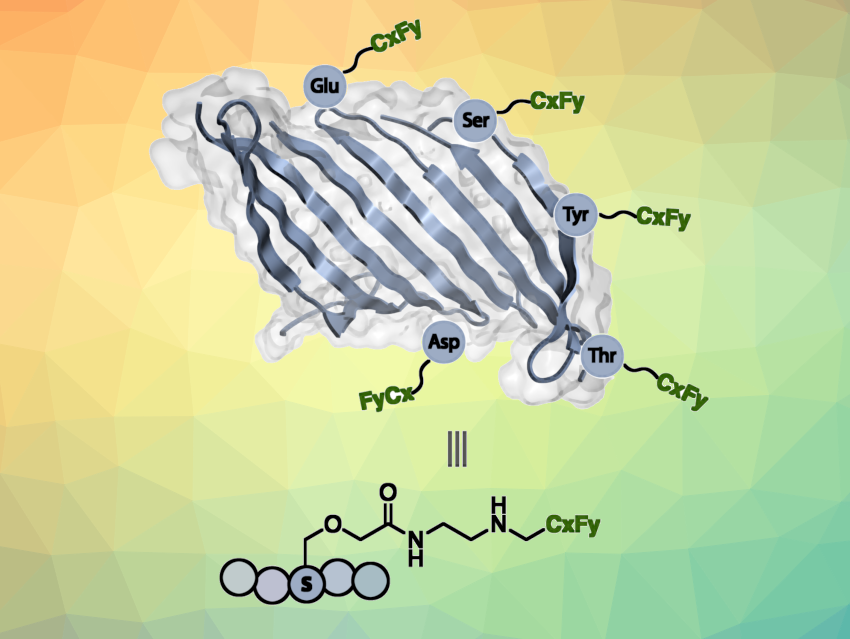
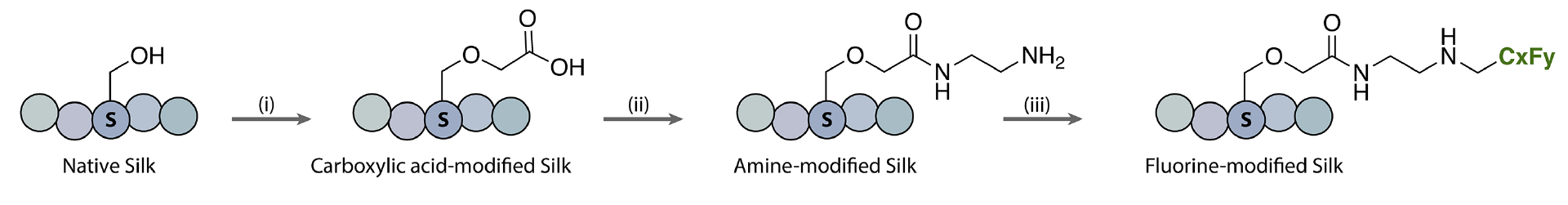
To mitigate these issues, Luke M. Davis, David L. Kaplan, Krishna Kumar, Tufts University, Medford, MA, USA, and colleagues have installed perfluorocarbon chains on the surface of silk fibroin to transform this usually water-soluble protein into a remarkably hydrophobic polymer—i.e., create “non-stick” silk. The team first modified hydroxyl and carboxylic acid groups to introduce primary amine functionalities. Iodonium salts containing the desired fluorinated substituent (e.g., CF3, C3F7, C7F15, C8F17) were then reacted with the primary amines to form new C–N bonds and graft the fluorocarbon chains onto the silk protein.
The water-repelling and anti-fouling properties were assessed by measuring water contact angles (WCAs). Native silk films are hydrophilic with WCAs of ca. 60°. The silk material with C8F17 substituents attained a remarkably large WCA of 125°, which indicates that it is even more hydrophobic than Teflon. The water uptake of thermoplastic molded silk bars was also dramatically decreased, which is promising for the use of such modified silk materials in biological systems. According to the researchers, this approach could be useful, e.g., in the fabrication of high-performance biomedical devices.
- Towards Non‐Stick Silk: Tuning the Hydrophobicity of Silk Fibroin Protein,
Julia N. Fountain, Morgan J. Hawker, Vittorio Montanari, Lauren Hartle, Junqi Wu, Jugal Kishore Sahoo, Luke M. Davis, David L. Kaplan, Krishna Kumar,
ChemBioChem 2022.
https://doi.org/10.1002/cbic.202200429