Computational methods can play an important role in the chemical sciences. Thanks to molecular simulations, it is often possible to obtain information on chemical reactions at a much lower cost compared with experiments. In this context, it is crucial to find suitable methods for the problem at a hand. They need to be as accurate as necessary, but as fast as possible.
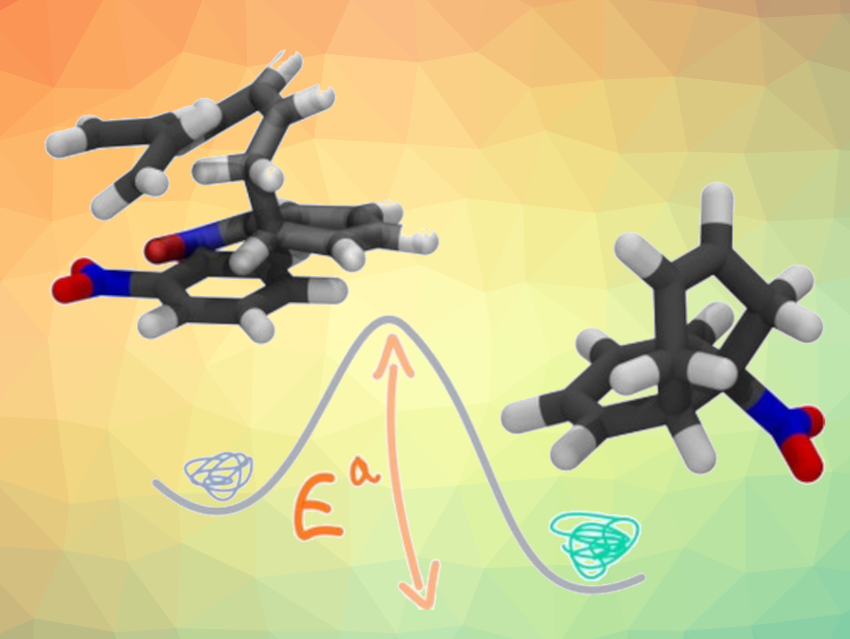
Jean-Philip Piquemal, Riccardo Spezia, Sorbonne Université, Paris, France, and colleagues have searched for relatively low-cost computational methods that can predict the characteristics of Diels–Alder reactions. They compared results obtained from density functional theory (DFT) calculations with small, “affordable” basis sets (a set of functions used to represent the electronic wave function) with very accurate but computationally expensive methods for a set of “small”, prototypical reactions. They found that fast DFT approaches can correctly predict the diastereoselectivity in Diels–Alder reactions with complex strained cyclic allene dienophiles, which is challenging due to the small energy differences between the endo and exo approaches.
Based on their results, the researchers suggest several DFT functionals that, together with a small basis set (6-31G) and dispersion corrections (corrections that account for attractive forces caused by fluctuations in electron density), can provide a good description of Diels–Alder reactions, in particular in terms of diastereoselectivity. These reliable, but cost-effective approaches could allow, for example, the simulation of large systems.
- Efficient and Accurate Description of Diels‐Alder Reactions Using Density Functional Theory,
Daniele Loco, Isabelle Chataigner, Jean‐Philip Piquemal, Riccardo Spezia,
ChemPhysChem 2022.
https://doi.org/10.1002/cphc.202200349



EXCELLENT PAPER