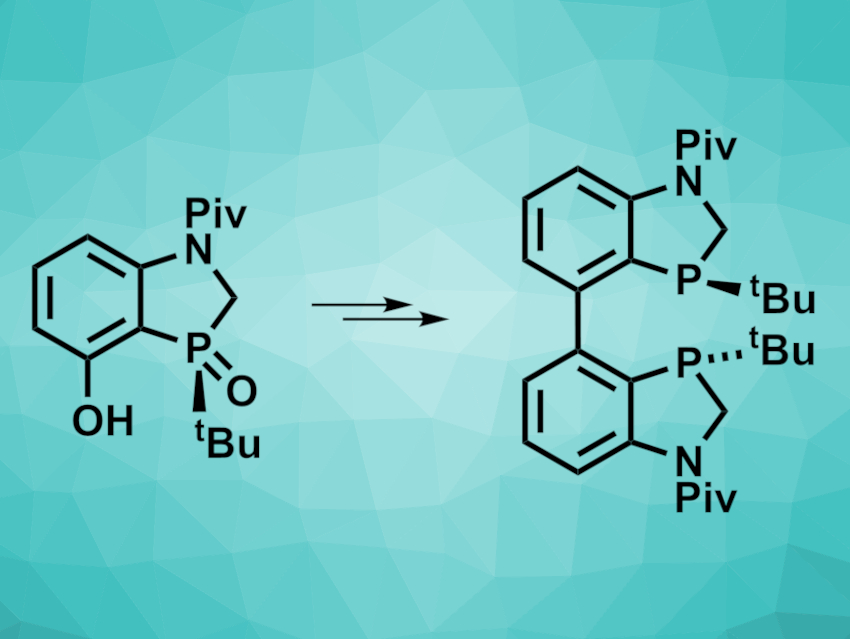
Chiral ligands are important in asymmetric catalysis. pivZPhos (pictured above on the right), for example, can be used as a P-chiral ligand in copper-catalyzed asymmetric hydrogenations or aminoborations. It is generally prepared from a dihydrobenzoazaphosphole precursor (pictured above on the left). This precursor is usually obtained using chiral column separation, which is a variant of column chromatography with a chiral stationary phase. This process is often expensive and time-consuming and can be challenging to scale up.
Yongda Zhang, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA, and colleagues have developed a more practical synthesis of this P-chiral dihydrobenzoazaphosphole precursor. The team used a chiral chemical resolution step with (1S,2S)-diaminocyclohexane as the resolution reagent (instead of the usual chiral column separation) to resolve a racemic mixture of the desired precursor. They combined the precursor with (1S,2S)-diaminocyclohexane in acetonitrile to obtain a solid adduct of the desired enantiomer with the diaminocyclohexane. The solid provides an enantiomeric ratio of about 94:6.
After further optimization of the stoichiometry and the reaction time, the researchers achieved enantiomeric ratios of over 99:1 and yields of 68–70 %. When the solid was treated with HCl in water, the desired precursor was released. It can be converted to pivZPhos in three steps. According to the researchers, the developed approach could allow for the production of the ligand precursor on a large scale.
- A Practical Method for the Large-Scale Preparation of the P-Chiral Dihydrobenzoazaphosphole Core by Chemical Resolution,
Jean-Nicolas Desrosiers, Yibo Xu, Stephanie C. Kosnik, Bo Qu, Kendricks S. Lao, Chris H. Senanayake, Yongda Zhang,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.2c00495