Pyridine rings are frequently found in natural products, pharmaceuticals, or agrochemicals. The direct modification of pyridine itself would be particularly attractive for the synthesis of structurally complex, biologically active pyridine derivatives. However, the direct, regioselective C–H functionalization of pyridine is challenging and commonly requires prefunctionalized starting materials with tailored protecting groups.
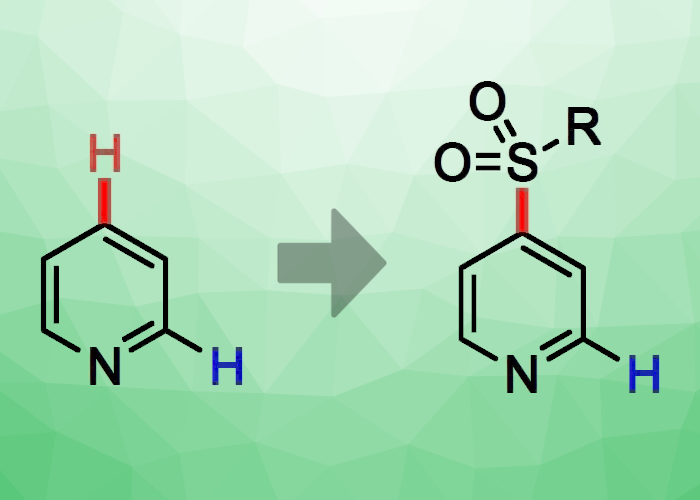
Marius Friedrich and Georg Manolikakes, Technical University Kaiserslautern, Germany, have developed a new, base-mediated approach for the direct, C4-selective sulfonylation of pyridine (pictured). The protocol involves a well-established preactivation of pyridine with triflic anhydride (TfO2), followed by a base-mediated addition of a nucleophlic sulfinic acid salt, such as sodium para-toluenesulfinate (NaTs), and an elimination/rearomatization process. The team used CHCl3 as the solvent and N-methylpiperidine as the base.
Under these conditions, the researchers achieved high regioselectivities for sulfonylation at C4. The selected base helps to steer the positioning of the sulfonyl substituent. The team was able to extend the method to allow the modular construction of sulfonylated pyridines. For this, they used, e.g., the sulfur dioxide surrogate 1,4-diazabicyclo[2.2.2]octane bis(sulfur dioxide) (DABSO), phenyl lithium, and the activated pyridinium triflate to prepare a C4-sulfonylated pyridine from three separate building blocks. The researchers aim to extend the scope of this method both to other N-heteroaromatics and other types of nucleophiles in future work.
- Base‐mediated C4‐selective C‐H‐sulfonylation of pyridine,
Marius Friedrich, Georg Manolikakes,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200915



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)