A limitation of current anticancer chemotherapy drugs is that they also target healthy cells, leading to side effects. Identifying compounds that specifically address enzymes that are much more abundant in cancer cells than normal cells is, therefore, important.
Thomas Helleday, Karolinska Institute, Stockholm, Sweden, and The University of Sheffield, UK, Pål Stenmark, Stockholm University, Sweden, and colleagues work towards developing drugs that inhibit methylenetetrahydrofolate dehydrogenase 2 (MTHFD2), an enzyme that is overexpressed in cancer cells but not in healthy cells. Selectively targeting MTHFD2 could result in cancer treatments with fewer side effects. Unfortunately, the strongest MTHFD2 inhibitors also target the related enzymes MTHFD1 and MTHFD2L, which are found in healthy cells. Improved knowledge about the structures of these enzymes (and their complexes) could help with the development of more selective drug candidates.
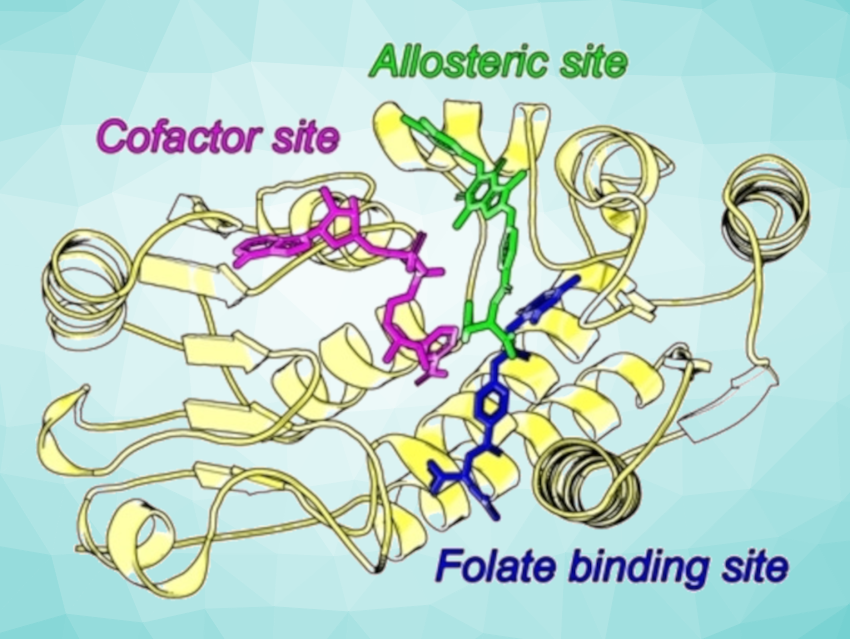
The team used X-ray crystallography at a synchrotron facility to solve the first structure of MTHFD2L and its complex with a potent inhibitor (TH7299). They then performed detailed structural comparisons of MTHFD2L with known structures of MTHFD2 and MTHFD1 and analyzed specific areas that could be targeted by inhibitors, including the folate, cofactor, and allosteric binding sites (pictured). For example, an interaction between a tyrosine residue of MTHFD2L and a glutamate unit of TH7299 is also present at the same position in MTHFD1, but MTHFD2 has a different residue at this site. The obtained information could guide future research aimed at developing more specific MTHFD2 inhibitors.
- The First Structure of Human MTHFD2L and Its Implications for the Development of Isoform‐Selective Inhibitors,
Emma R. Scaletti, Robert Gustafsson Westergren, Yasmin Andersson, Elisee Wiita, Martin Henriksson, Evert J. Homan, Ann‐Sofie Jemth, Thomas Helleday, Pål Stenmark,
ChemMedChem 2022.
https://doi.org/10.1002/cmdc.202200274