The heavier analogues of azenes (RE=ER with E = P, As, Sb, Bi) are interesting research targets. There are examples of diphosphenes, diarsenes, distibenes, and dibismuthenes, which are stabilized using bulky substituents. These compounds could be reduced to give radical anions of the type [RE=ER]•–. This is known for diphosphenes, rarely observed in distibenes, and has been found for one dibismuthene. An isolable diarsene radical anion had not been reported so far.
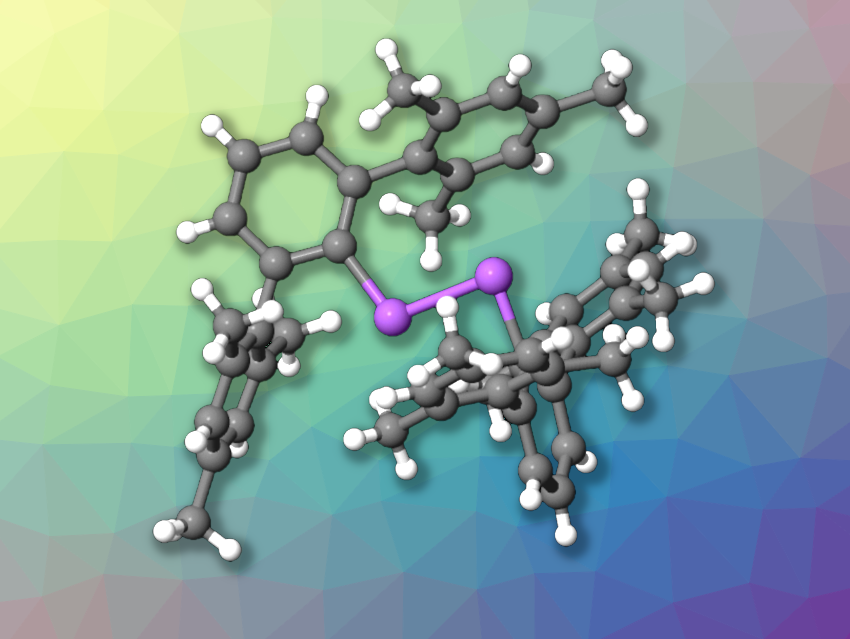
Christian Hering-Junghans, Leibniz Institute for Catalysis, Rostock, Germany, C. Gunnar Werncke, University of Marburg, Germany, and colleagues have isolated the first diarsene radical anion (structure pictured). The team started from the neutral diarsene (MesTerAs)2 (MesTer = 2,6-Mes2-C6H3), which has a bulky, terphenyl-based substituent. This precursor was prepared from MesTerAsCl2 via a reduction using Zn and PMe3.
The neutral diarsene (MesTerAs)2 was then treated with KC8 in tetrahydrofuran (THF), using 18-crown-6 (18c6) to capture the potassium ions. The team obtained crystals of [K(18c6)(THF)][(MesTerAs)2] with a diarsene radical anion in a yield of 28 %. The salt degrades within several minutes to hours to give (MesTerAs)2. The researchers also used the iron(I) silylamide [K(18c6)][Fe(NR2)2] (R = SiMe3) as an alternative reductant and obtained solvent-free [K(18c6)][(MesTerAs)2] in an improved yield of 63 %.
Both products were characterized using X-Ray diffraction analysis, UV/Vis and EPR spectroscopy, as well as computational methods. The results are consistent with a radical character centered at the As atoms. The team also performed preliminary reactivity studies, which show that the diarsene radical anion can act as a one-electron reductant.
- A Diarsene Radical Anion,
Grégoire Sieg, Malte M.F. Fischer, Fabian Dankert, Jan-Erik Siewert, Christian Hering-Junghans, Gunnar Werncke,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc03237f