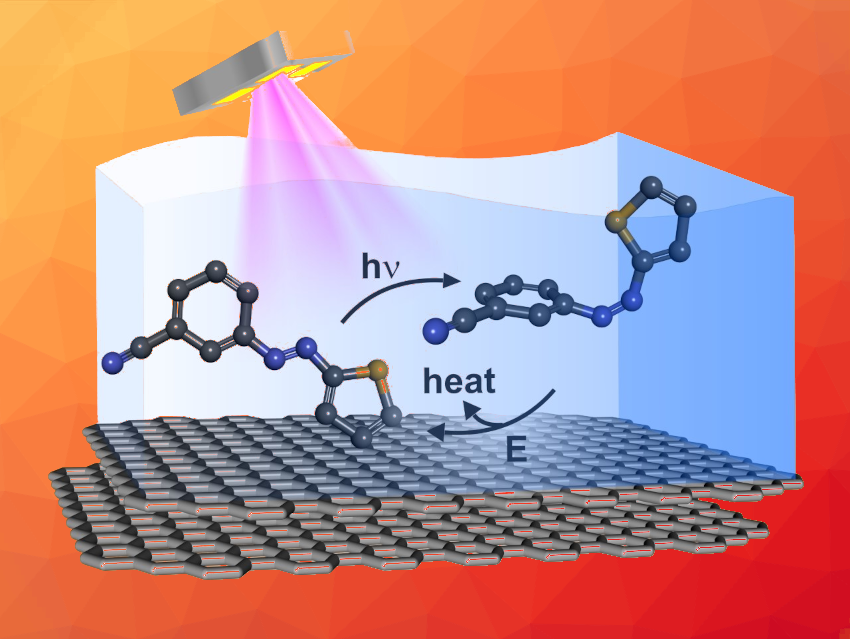
Molecular solar thermal systems (MOSTs) combine solar energy conversion, storage, and release in a simple molecular approach. The concept is based on molecular photoswitches, which are converted photochemically to a metastable high-energy state. Later, the stored energy can be released on demand in form of heat. For the implementation of MOST systems into operational devices, it is, however, important to control the energy release with excellent cyclability.
Olaf Brummel, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, and colleagues have demonstrated that this type of control is possible using an azothiophene-based MOST system with an electrochemical trigger for the energy release. The team studied the electrochemically triggered back-reaction from the energy-rich (Z)-3-cyanophenylazothiophene to its lower-energy (E)-isomer using highly oriented pyrolytic graphite (HOPG) as the working electrode (simplified structures pictured). For the photochemical conversion from the (E)-isomer to the (Z)-isomer, a high-power LED was used as a UV light source.
The researchers found that the rate of the back-conversion reaction can be tuned by the electrode potential within two orders of magnitude, providing control over the energy release. The MOST system also provides excellent cyclability. After 100 cycles, the photochemical conversion is still quantitative, and the electrochemically triggered back-reaction reaches 94 % of the original conversion level.
- Electrochemically Triggered Energy Release from an Azothiophene‐based Molecular Solar Thermal System,
Evanie Franz, Anne Kunz, Nils Oberhof, Andreas H. Heindl, Manon Bertram, Lukas Fusek, Nicola Taccardi, Peter Wasserscheid, Andreas Dreuw, Hermann Wegner, Olaf Brummel, Jörg Libuda,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202200958