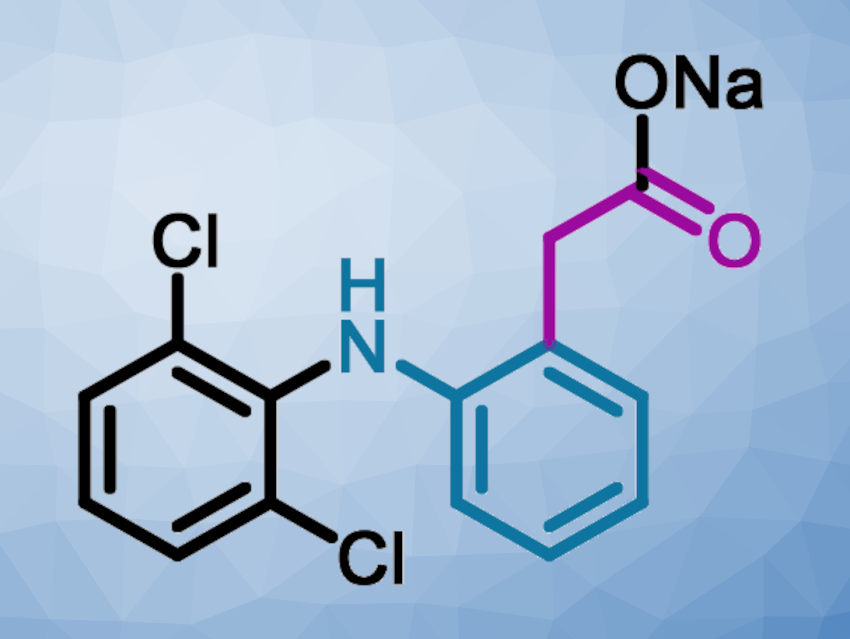
Diclofenac sodium (pictured above) is a widely-used non-steroidal anti-inflammatory drug (NSAID). The current industrial method for the synthesis of diclofenac sodium uses 2,6-dichloro-N-diphenylaniline as a key intermediate, which is obtained via a Smiles rearrangement followed by an amide hydrolysis. This hydrolysis removes a C2 unit as waste in the form of 2-hydroxyacetic acid sodium salt, and afterward, a new C2 group is introduced using toxic chloroacetyl chloride. Thus, the amide hydrolysis is an extra step needed in this traditional batch process that causes waste and reduces efficiency.
Dang Cheng, Fener Chen, Shanghai Engineering Research Center of Industrial Asymmetric Catalysis of Chiral Drugs and Fudan University, Shanghai, China, and colleagues have developed an integrated six-step continuous flow process for the synthesis of diclofenac sodium starting from aniline and chloroacetic acid (pictured below), which form an amide. The team used a cascade etherification/Smiles rearrangement in which the resulting 2-chloro-N-phenylacetamide and 2,6-dichlorophenol are converted to a hydroxyacetyldiphenylamine derivative, skipping the formation of 2,6-dichloro-N-diphenylaniline. A chlorination, followed by an intramolecular Friedel-Crafts cyclization and hydrolysis then gave the desired product.
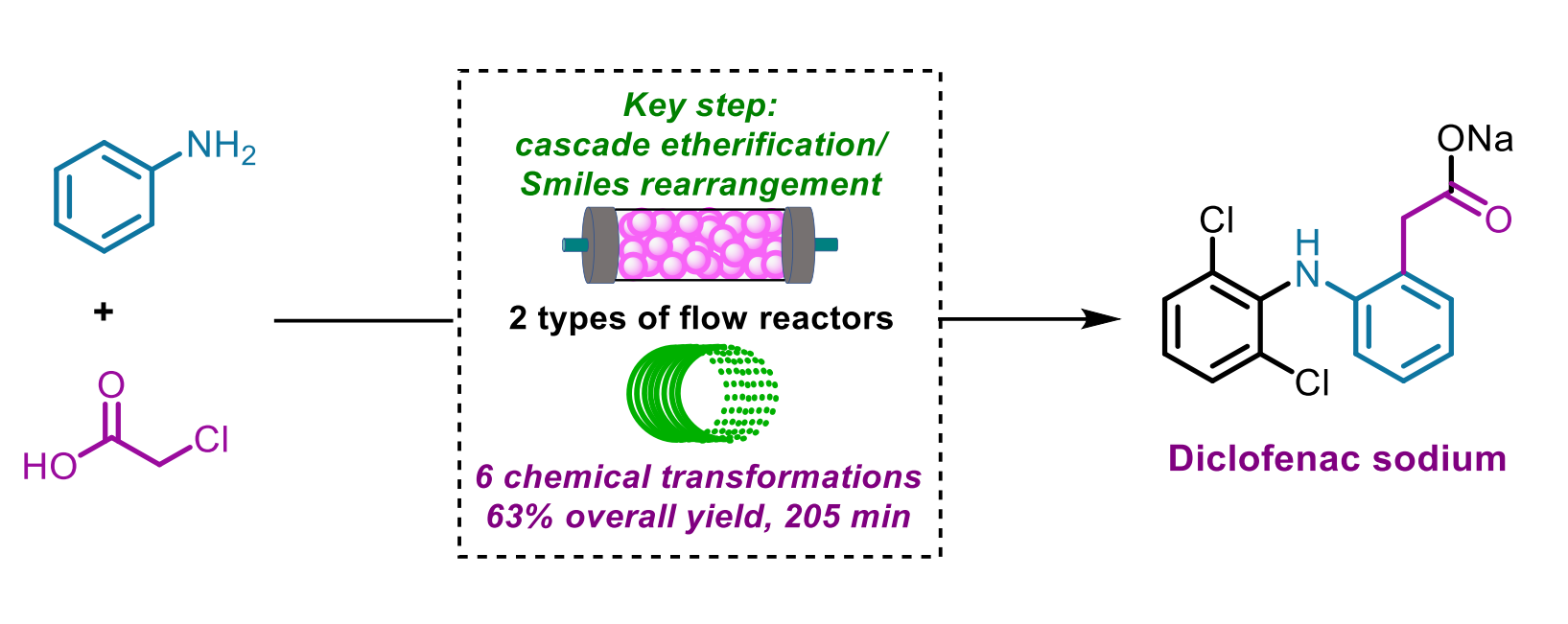
The process gives an overall isolated yield of 63 % with a total residence time of less than 3.5 h. Compared with the traditional batch synthesis, the key features of this flow approach to the synthesis of diclofenac sodium are high efficiency, high atom and step economy, short reaction times, improved sustainability, and simple and automatic operations. This could indicate potential for industrial manufacturing.
- Six‐Step Continuous Flow Synthesis of Diclofenac Sodium via Cascade Etherification/Smiles Rearrangement Strategy: Tackling the Issues of Batch Processing,
Lulu Wang, Minjie Liu, Meifen Jiang, Li Wan, Weijian Li, Dang Cheng, Fener Chen,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201420