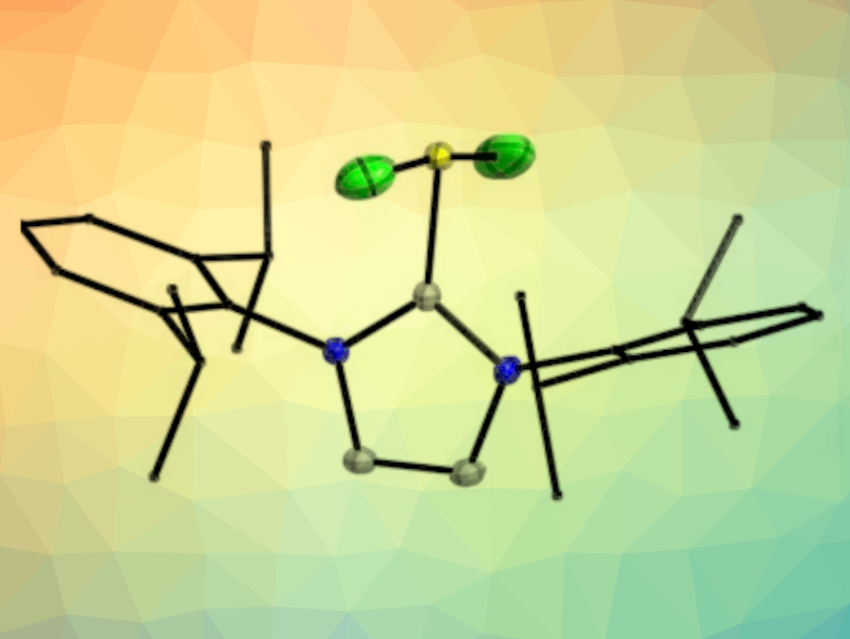
The chalcogen difluorides SF2 and SeF2 are extremely reactive species that can generally only be isolated by matrix techniques. Selenium tetrafluoride (SeF4) is a very corrosive and toxic liquid, the preparation of which usually involves gaseous SF4 and SeO2 in an autoclave.
Marian Olaru, Jens Beckmann, University of Bremen, Germany, Stefan Mebs, Free University Berlin, Germany, and colleagues have prepared donor–acceptor complexes between SF2, SeF2, and SeF4 and an N-heterocyclic carbene (1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, IPr) that were isolated as the crystalline solids IPrSF2 (pictured), IPrSeF2, and IPrSeF4.
The complexes were prepared via the stoichiometry-controlled fluorination of imidazol-2-thione or the corresponding selenone using either one or two equivalents of solid XeF2. This approach avoids the generation of the highly unstable and/or toxic parent chalcogen fluorides. When the team tried to extend this chemistry to tellurium, they obtained the metastable ionic complex [IPr2TeF3][TeF5], which slowly converted into the abnormal NHC complex aIPrTeF4 (aIPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-4-ylidene, i.e., the NHC binds via a carbon atom in its backbone).
The products were fully characterized using X-ray crystallography and NMR spectroscopy. The electronic structures of the donor–acceptor complexes were studied by density-functional theory (DFT) calculations. The solid complexes IPrSF2, IPrSeF2, and IPrSeF4 can be regarded as “tamed” synthons of their highly reactive parent chalcogen fluorides.
- Donor Acceptor Complexes between the Chalcogen Fluorides SF2, SeF2, SeF4 and TeF4 and an N‐Heterocyclic Carbene,
Pascal Komorr, Marian Olaru, Emanuel Hupf, Stefan Mebs, Jens Beckmann,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201023