Diaryl ethers are commonly found, e.g., in natural products, pharmaceuticals, or agrochemicals. There are some examples of biosynthetic transformations that give diaryl ethers, usually involving oxidation reactions. One example of nonoxidative diaryl ether formation involves the fungal nonreducing polyketide synthase (nrPKS) AN7909.
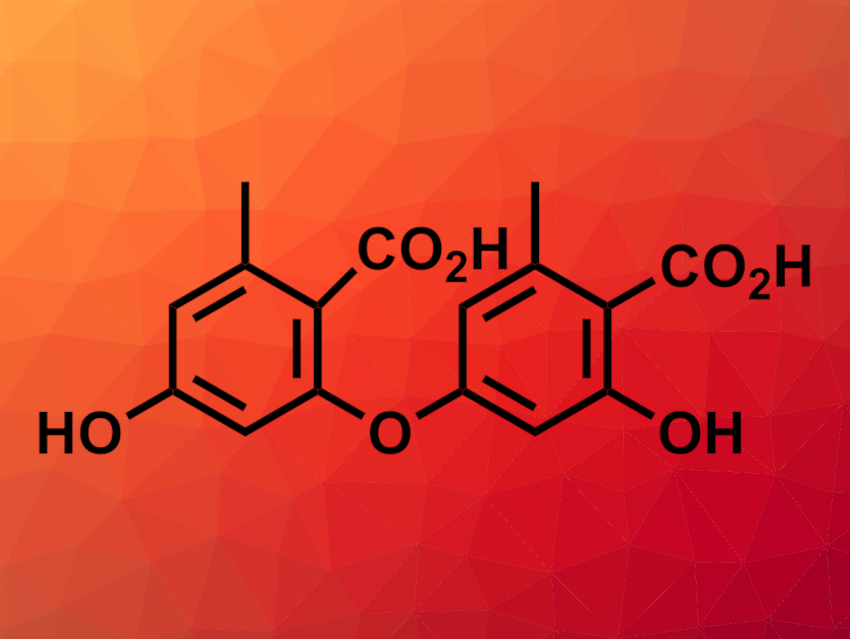
Xiao Zhang, Yi Zou, Southwest University, Chongqing, China, and colleagues have found that the thioesterase domain from AN7909 catalyzes diaryl ether formation through a series of successive steps, including esterification, a Smiles rearrangement, and hydrolysis. The team purified AN7909 from the yeast Saccharomyces cerevisiae and also prepared its thioesterase domain separately. Both the enzyme and the thioesterase domain alone catalyzed the formation of the diaryl ether diorcinolic acid (pictured).
Using different substrates, the team investigated the catalytic activity of the thioesterase domain and the mechanism of the ether formation. They found that the thioesterase domain is critical for the synthesis of diorcinolic acid and that it catalyzes four successive steps: chain transfer, ester bond formation, ester-ether bond exchange, and hydrolysis. According to the researchers, this represents the first example of biosynthetic diaryl ether formation that does not proceed via an oxidative coupling or an oxidative rearrangement.
- Diaryl Ether Formation by a Versatile Thioesterase Domain,
Qian Wei, Ze-Ping Wang, Xiao Zhang, Yi Zou,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c02813