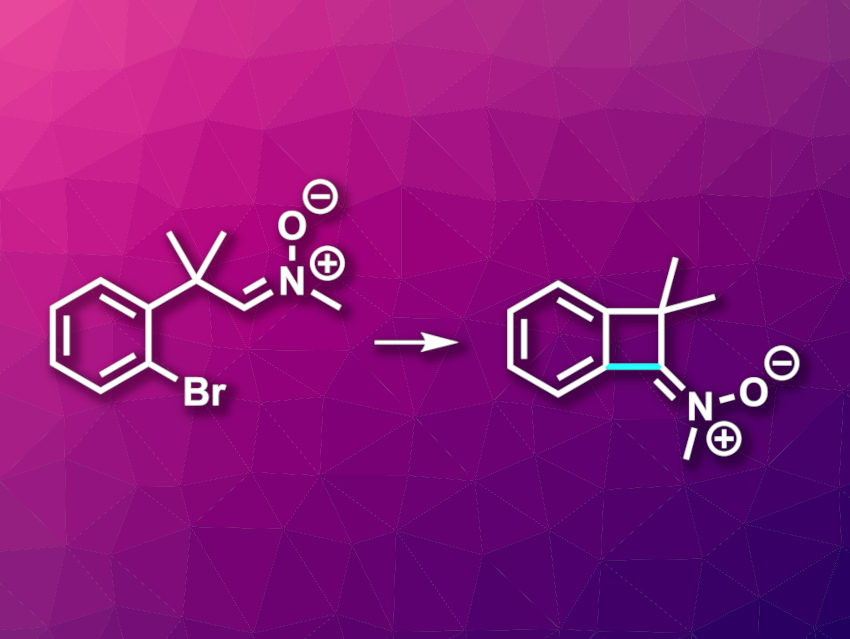
Organic compounds with strained, four-membered rings can be useful building blocks in organic synthesis. Benzocyclobutenes, for example, which consist of fused benzene and cyclobutene rings, can undergo a variety of useful transformations. Incorporating a nitrone group (R2C=N+(R’)–O–) might expand this range further.
Jakub Brześkiewicz and Rafał Loska, Polish Academy of Sciences, Warsaw, Poland, have developed the first protocol for the synthesis of benzocyclobutenone-derived ketonitrones (pictured). The team started from bromoaryl-substituted aldonitrones, which were subjected to a palladium-catalyzed intramolecular C(sp2)–H functionalization to close the four-membered ring. The aldonitrone substrates could be readily obtained from (2-bromophenyl)acetonitriles. The researchers used Pd(OAc)2 as a catalyst, 1,2-bis(diphenylphosphino)ethane (dppe) as a ligand, Cs2CO3 as a base, and toluene as the solvent. The reactions were performed at 100 °C.
The desired benzocyclobutenitrones were obtained in mostly high yields. The reaction tolerates a broad range of electron-withdrawing and electron-donating substituents at the benzene ring, as well as different substituents in α-position to the nitrone group. The products can be used in further transformations, e.g., 1,3-dipolar cycloadditions or nucleophilic additions.
- Palladium-Catalyzed Access to Benzocyclobutenone-Derived Ketonitrones via C(sp2)–H Functionalization,
Jakub Brześkiewicz, Rafał Loska,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01317