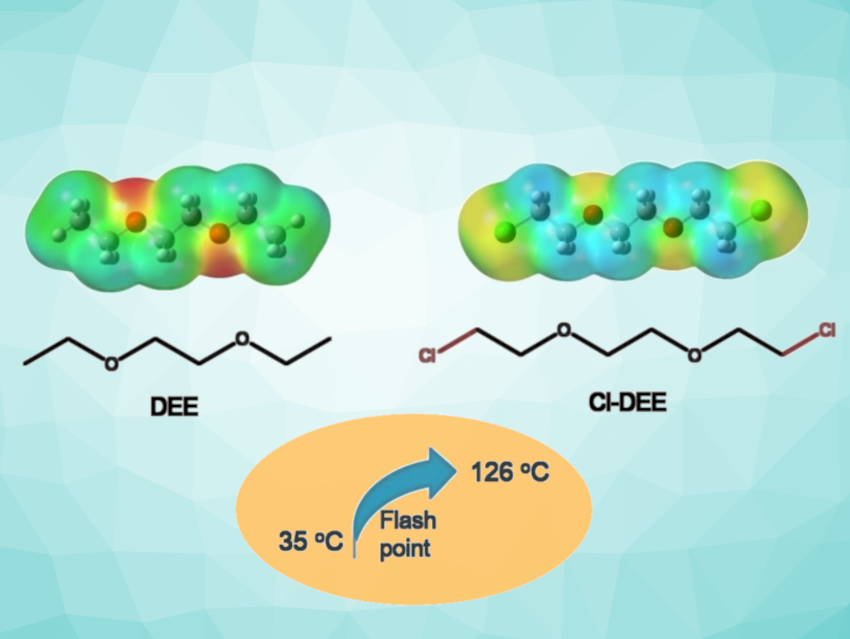
Ether-based electrolytes with high reduction stability are important in the development of batteries. However, their generally insufficient stability toward oxidation and high flammability restrict applications in high-voltage lithium-metal batteries.
Inspired by the flame-retarding mechanism of chlorine in fire-extinguishing agents, Xiaodi Ren, University of Science and Technology of China, Hefei, and colleagues have used a chloroether, i.e., 1,2-bis(2-chloroethoxy) ethane (Cl-DEE, pictured), as the base solvent in a localized high-concentration electrolyte (LHCE) system. The electrolyte system contained lithium bis(fluorosulfonyl)imide (LiFSI), Cl-DEE, and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether (TTE). The additional chlorine groups improve the oxidation stability and turn the highly flammable parent molecule DEE (pictured) into a flame retardant, increasing the flashpoint from 35 °C to 126 °C
The team found that the formulated chloroether electrolyte allows for stable battery operation at an ultrahigh voltage of up to 4.7 V. LiF/LiCl-enriched interphases formed on the cathode and the anode effectively suppress undesired side reactions for better cycling life. The molecular design concept of chlorine substitution in this work could provide a useful approach to developing safe lithium-metal batteries with high energy density.
- Intrinsic Nonflammable Ether Electrolytes for Ultrahigh‐Voltage Lithium Metal Batteries Enabled by Chlorine Functionality,
Lijiang Tan, Shunqiang Chen, Yawei Chen, Jiajia Fan, Digen Ruan, Qingshun Nian, Li Chen, Shuhong Jiao, Xiaodi Ren,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202203693