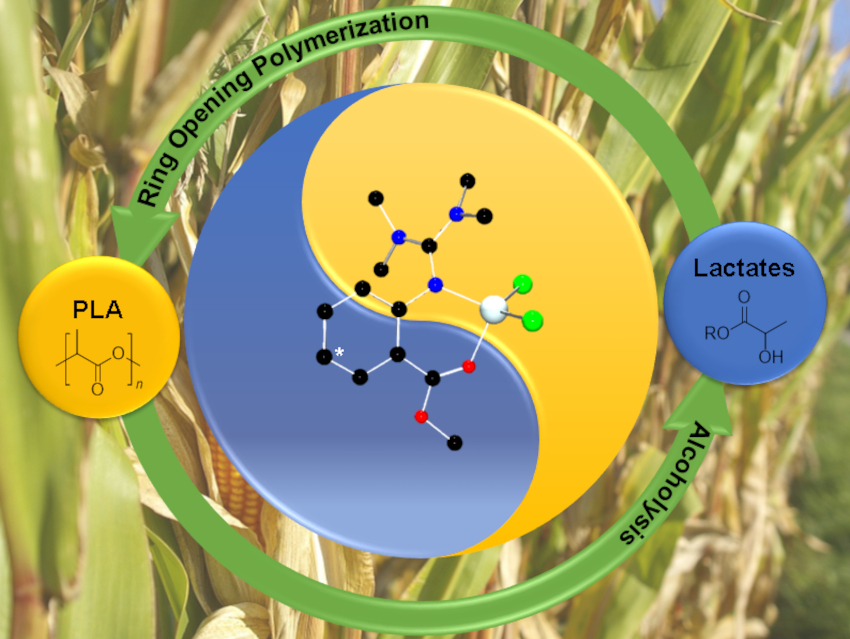
Polylactide (PLA) is a renewable, biodegradable polyester accessible via metal-catalyzed ring-opening polymerization (ROP) of lactide. It could be a good candidate to substitute common fossil-based, non-degradable plastics and help to fight the pollution caused by these polymers. However, current industrial production requires the use of the toxic catalyst tin octanoate and costly monomer production via fermentation. Therefore, efficient recycling is important to exploit the full potential of PLA.
Sonja Herres-Pawlis and colleagues, RWTH Aachen University, Germany, have investigated the chemical recycling of PLA via methanolysis, transforming the polymer to methyl lactate in anhydrous tetrahydrofuran (THF). The team used guanidine carboxy Zn complexes (pictured below) to promote the reaction. Using the unsubstituted catalyst C2, a polyester conversion of 41 % was reached after 5 h at 60 °C. Introducing an electron-donating NMe2 group at the ligand’s backbone (C3) caused an increase in catalyst activity, resulting in a PLA conversion of 72 %. Introducing an electron-density-withdrawing group to the ligand (C1) decreases the conversion.

The team tested the optimized catalyst C3 for industrially relevant parameters such as process scalability. The team found that scale-up of the reaction and catalyst recycling are both possible. The catalyst can also promote selective PLA degradation from a mixed plastics feed. According to the researchers, guanidine carboxy Zn catalysts could be a useful tool to create a circular (bio)plastics economy.
- Guanidine Carboxy Zinc Complexes for the Chemical Recycling of Renewable Polyesters,
Martin Fuchs, Marcel Walbeck, Eveline Jagla, Alexander Hoffmann, Sonja Herres-Pawlis,
ChemPlusChem 2022.
https://doi.org/10.1002/cplu.202200029