Tuberculosis (TB) is a potentially deadly bacterial disease. To combat drug-resistant TB, novel classes of active substances that act on new drug targets are needed.
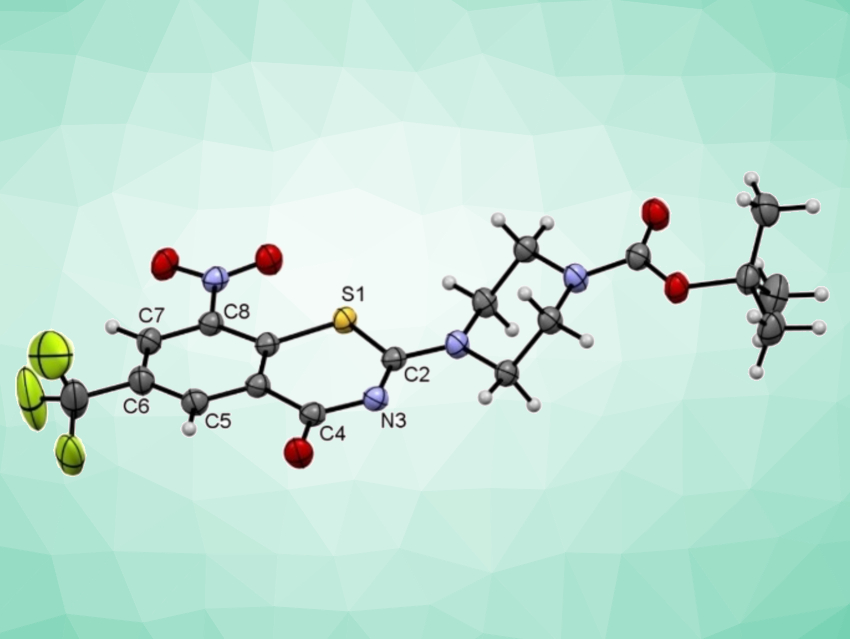
8-Nitrobenzothiazinones (BTZs, example pictured above) constitute a highly effective antimycobacterial class of substances that inhibit the growth of Mycobacterium tuberculosis (Mtb), even in the nanomolar concentration range. A wide variety of BTZ derivatives has been prepared and investigated in recent years using different synthetic routes. However, these routes have drawbacks such as a need for toxic reagents, which can be problematic for pharmaceuticals manufacturing and the environment.
Peter Imming, Martin Luther University Halle-Wittenberg, Halle, Germany, and colleagues have developed a new synthesis of BTZs that avoids the use of toxic reagents and is suitable for good manufacturing practice (GMP) requirements in the pharmaceutical industry. The team started from 2-chloro-3-nitro-5-(trifluoromethyl)benzoic acid (pictured below), which was activated with thionyl chloride. The resulting intermediate was reacted with a thiourea derivative (X = S) to give the desired thiazinone ring system.

Using this approach, 36 different BTZ derivatives were prepared and their activity against Mtb was determined. The synthetic procedure also allows the replacement of the sulfur atom in the thiazinone ring system with oxygen or nitrogen (X = O,N) to give benzoxazinone and quinazolinone systems.
- Efficient Synthesis of Benzothiazinone Analogues with Activity against Intracellular Mycobacterium tuberculosis,
Adrian Richter, Gagandeep Narula, Ines Rudolph, Rüdiger W. Seidel, Christoph Wagner, Yossef Av‐Gay, Peter Imming,
ChemMedChem 2021.
https://doi.org/10.1002/cmdc.202100733