The chemistry of transuranium elements is understudied compared with the rest of the periodic table due to their radioactivity and limited availability. However, it is important to study these elements, especially neptunium, because of their prominence in spent nuclear fuel. Although neptunium’s aqueous chemistry has been developed to a certain degree, the non-aqueous chemistry of the element is still not well understood.
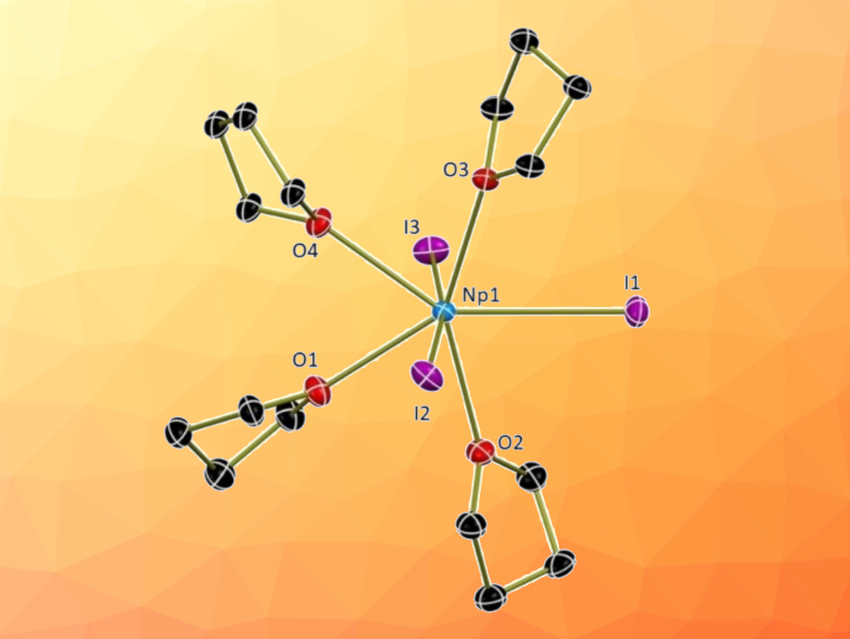
Suzanne C. Bart and colleagues, Purdue University, West Lafayette, IN, USA, have developed non-aqueous methods to synthesize an Np4+ starting material and two new Np3+ compounds. NpO2 was first converted to NpCl4(DME)2 (DME = dimethoxyethane) by first dissolving it in concentrated hydrochloric acid with added hydrofluoric acid. After drying to a residue and dissolving in anhydrous DME, SiMe3Cl was added to obtain the desired product. NpCl4(DME)2 was then reduced to the trivalent state using KC8 (pictured below), forming NpCl3(THF)x (THF = tetrahydrofuran). This intermediate was converted to NpI3(THF)4 and NpBr3(THF)4 through a halide exchange reaction.

All compounds were analyzed using 1H NMR, crystallography, and electronic absorption spectroscopy. In summary, three convenient synthetic routes to neptunium salts were reported, resulting in the compounds NpCl4(dme)2, NpI3(THF)4, and NpBr3(THF)4. These straightforward reaction pathways could help to advance the field of non-aqueous trivalent actinide chemistry.
- Synthesis of Non‐Aqueous Neptunium(III) Halide Solvates from NpO2,
Megan A. Whitefoot, Diana Perales, Matthias Zeller, Suzanne C. Bart,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202103265