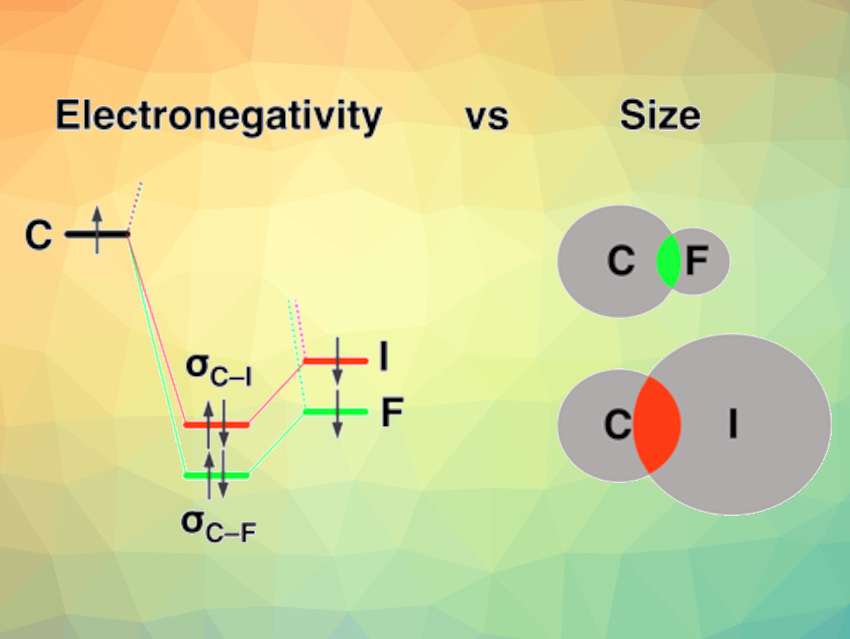
The chemical bond is a key concept in chemistry. In general, X–Y bonds become stronger as the electronegativity difference across the bond increases. In a paradigm shift, theoretical chemists have found that this apparent correlation between the electron-pair bond strength and the electronegativity difference across the bond is not operative for some surprisingly simple bonds.
F. Matthias Bickelhaupt, Vrije Universiteit Amsterdam and Radboud University, Nijmegen, both The Netherlands, and colleagues have used density functional theory (DFT) calculations to analyze X–Y element–element bonds across the periodic table. Along a period, e. g., from C−C to C−F, bonds get stronger because the electronegativity difference across the bond increases. However, down a period, the team’s findings were surprising. From C–F to C–I, for example, bonds become weaker because of higher steric Pauli repulsion as the atom size increases—not because of the decreasing electronegativity difference (as “traditionally” believed). The team showed that the opposite is true: Carbon−halogen bonds become weaker from C−F to C−I despite the orbital interaction becoming stronger because of better bond overlap.
Interestingly, the carbon–halogen bond series is a popular, but erroneous, example often used in textbooks for illustrating the oversimplified electronegativity model. The observed trend is not a matter of attraction, but of repulsion.
- The Chemical Bond: When Atom Size Instead of Electronegativity Difference Determines Trend in Bond Strength,
Eva Blokker, Xiaobo Sun, Jordi Poater, J. Martijn van der Schuur, Trevor A. Hamlin, F. Matthias Bickelhaupt,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202103544