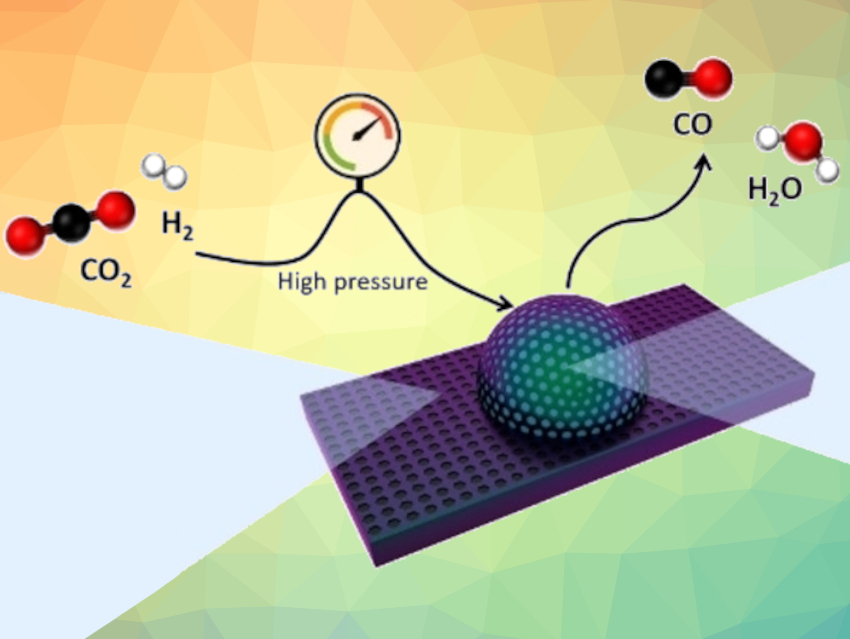
The conversion of CO2 into CO is an important step for the utilization of the greenhouse gas CO2 in clean fuels and value-added chemicals. CO is a much more reactive feedstock than CO2. However, the hydrogenation of CO2 usually gives byproducts such as methane. This hampers the production of CO and its conversion into compounds useful in industry, such as alcohols or long-chain hydrocarbons.
Liane M. Rossi, Universidade de São Paulo, Brazil, and colleagues have developed a catalyst based on carbon, nickel, and zinc that promotes the selective hydrogenation of CO2 into CO via the reverse water gas shift (RWGS) reaction. The methanation reaction of CO2/CO, which is usually favored over Ni catalysts, was completely suppressed. The team used the pyrolysis of zeolitic imidazolate framework-8 (ZIF-8) loaded with different amounts of Ni2+ to obtain Ni−Zn carbide (Ni3ZnC) embedded in N-doped nanocarbon (pictured).

The catalyst reached CO selectivities close to 100 %. It is selective towards CO under a wide range of reaction conditions, including high-pressure conditions that are relevant for subsequent CO conversion processes. The researchers propose that the catalyst can carry out CO2 and H2 activation through an intermediate that is readily desorbed into the gas phase. The short residence time of this weakly adsorbed intermediate prevents its hydrogenation to methane.
- Zeolitic‐Imidazolate Framework Derived Intermetallic Nickel Zinc Carbide Material as a Selective Catalyst for CO2 to CO Reduction at High Pressure,
Nágila E. C. Maluf, Adriano H. Braga, Maitê L. Gothe, Laís R. Borges, Gustavo A. S. Alves, Renato V. Gonçalves, János Szanyi, Pedro Vidinha, Liane M. Rossi,
Eur. J. Inorg. Chem. 2021.
https://doi.org/10.1002/ejic.202100530