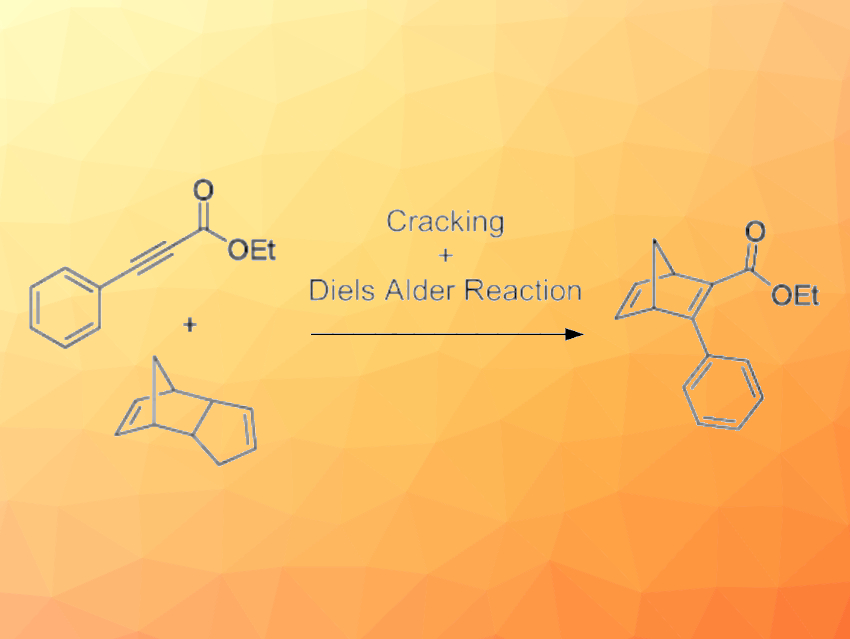
The norbornadiene (NBD)-quadricyclane (QC) photoswitch (pictured below on the right) could have applications in solar energy storage due to the large energy difference between the two isomers. For testing in devices, larger amounts of functionalized norbornadienes (NBDs) need to be prepared. The Diels–Alder reaction between an alkyne and cyclopentadiene is one approach to synthesizing NBDs. However, this method has some challenges related to the scale-up and usually requires the cracking of dicyclopentadiene before utilization.
Kasper Moth-Poulsen, Chalmers University of Technology, Gothenburg, Sweden, and colleagues have developed a protocol for synthesizing NBDs (pictured above) using a tubular flow reactor where in-situ cracking of dicyclopentadiene and the subsequent Diels–Alder reaction are done in one single step at 200 ˚C and 9 bar. The team used a stream containing a solution of the different alkynes and dicyclopentadiene in toluene in a flow chemistry setup with a back-pressure regulator (BPR), which ensures the system is pressurized and enables the use of higher temperatures than the ambient boiling point of the solvent.
 Six examples of NBDs were synthesized in good to excellent yields. The synthesis of one NBD was scaled up to produce 87 g in 9 h. The use of flow chemistry with in-situ dicyclopentadiene cracking provides a convenient technique for the fast and efficient large-scale synthesis of NBDs.
Six examples of NBDs were synthesized in good to excellent yields. The synthesis of one NBD was scaled up to produce 87 g in 9 h. The use of flow chemistry with in-situ dicyclopentadiene cracking provides a convenient technique for the fast and efficient large-scale synthesis of NBDs.
- Scalable Synthesis of Norbornadienes via in situ Cracking of Dicyclopentadiene using Continuous Flow Chemistry,
Jessica Orrego-Hernández, Helen Hölzel, Maria Quant, Zhihang Wang, Kasper Moth-Poulsen,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100795