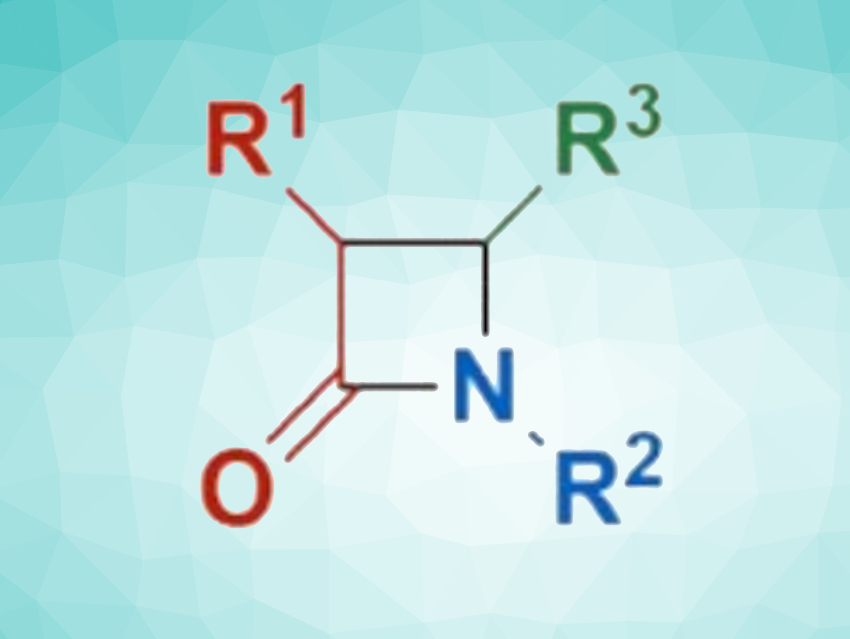
In the Staudinger synthesis, an imine reacts with a ketene via a thermal 2+2 cycloaddition to produce a β-lactam (pictured). However, the reaction has some drawbacks, such as the intrinsic instability of ketenes, the required low temperatures, and the need to form the imine derivatives before the reaction.
Andrea Basso, University of Genova, Italy, and colleagues have used a photochemical approach to assemble β-lactams directly from diazoketones as ketene precursors and amines/aldehydes as imine precursors in a three-component reaction. The team used different aliphatic and aromatic diazoketones, aromatic aldehydes, and amines as reactants and dichloromethane (DCM) as the solvent. The imines were first formed in the dark. Then, the diazoketones were activated under visible light to form ketenes, and the desired β-lactams were obtained.
The reactions proceeded with complete stereoselectivity in favor of the trans isomer. The isolated yields of the products obtained through the three-component reactions are lower than those of the corresponding reactions with preformed imines. However, according to the researchers, the lower yields are compensated for by the greater synthetic simplicity and the possibility of using the process in automated combinatorial synthesis.
- Ketene 3‐Component Staudinger Reaction (K‐3CSR) to β‐lactams: a new entry in the class of photoinduced multicomponent reactions,
Federica Minuto, Chiara Lambruschini, Andrea Basso,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100577