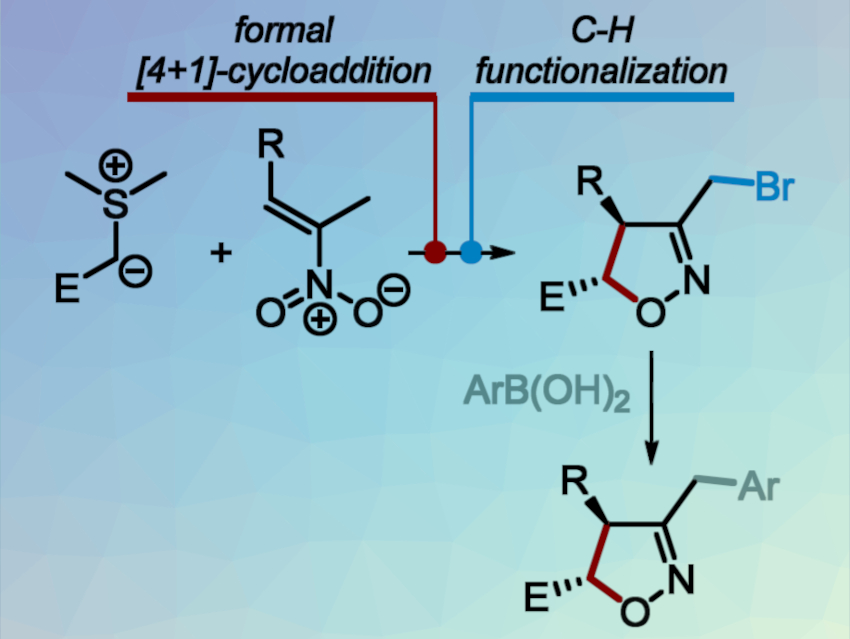
Isoxazolines are valuable building blocks for organic synthesis. They serve as starting materials for the preparation of hydroxyketones, aminoalcohols, pyrrolidines, and other medicinally important compounds. Common methods for isoxazoline synthesis use [3+2]-cycloadditions of nitrile oxides with alkenes, which can be tedious and non-regioselective for polysubstituted targets.
To overcome these issues, Andrey Tabolin, N. D. Zelinsky Institute of Organic Chemistry, Moscow, Russia, and colleagues have developed a modular, diastereoselective synthesis of 3,4,5-trisubstituted isoxazolines that is based on a [4+1]-cycloaddition route (pictured below). The team used a synthetic sequence that involves: 1) a [4+1]-annulation of sulfur ylides with nitroalkenes, 2) the functionalization of the side-chain methyl group by a one-pot silylation of isoxazoline-N-oxides, followed by treatment with ZnBr2, and 3) a Suzuki-Miyaura cross-coupling of bromomethyl-isoxazolines with boronic acids.

The process gives the desired trisubstituted isoxazolines in good yields with high diastereoselectivity. It allows the independent variation of the reaction partners and the introduction of a wide range of different substituents.
- Sequential formal [4+1]‐cycloaddition, C‐H functionalization and Suzuki‐Miyaura cross‐coupling for the synthesis of trisubstituted isoxazolines,
Pavel Yu. Ushakov, Alexey Yu. Sukhorukov, Sema L. Ioffe, Andrey Tabolin,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100313