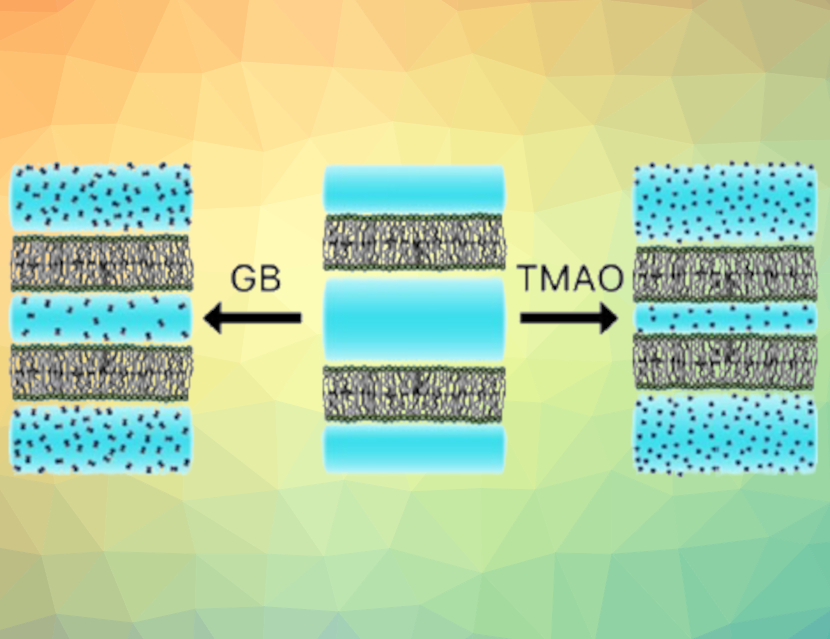
Osmolytes, small organic compounds such as glycine betaine (GB) and trimethylamine N-oxide (TMAO), can stabilize proteins against denaturation under raised temperatures and pressures. Some deep-sea animals accumulate osmolytes to maintain a certain osmotic pressure and counteract the effects of the high salt concentration and water pressure. While osmolyte effects on proteins have been well-established, less is known about the influence of these compounds, particularly GB, on lipid membranes.
Yuri Shakhman and Daniel Harries, The Hebrew University, Jerusalem, Israel, have studied the effects of the osmolyte GB on lipid bilayers. The researchers used dimyristoyl phosphatidylcholine (DMPC) bilayers as a model lipid system. These bilayers undergo self-assembly to form multilamellar vesicles. The team used small-angle X-ray scattering and vapor pressure osmometry, as well as measurements of the refractive index and the dielectric constant to study how GB modifies the interactions between lipid membranes and compare the effects with those of TMAO.
The researchers found that GB and TMAO bring the lipid bilayers closer together. This behavior is due to the compound’s partial exclusion from the space between the membranes, leading to an osmotic pressure that brings the membranes closer together (pictured). However, van der Waals attractive forces between membranes are significantly weakened in the presence of GB, so that the net effect of GB is smaller than that of TMAO. They also found that GB and urea can counteract each other over a wide range of GB-to-urea concentrations and at high total solute concentrations, leading to a minimal effect on interbilayer spacing. According to the researchers, these results support the hypothesis that mixtures of several osmolytes can achieve minimal net impact on biomacromolecular stability, while still counteracting osmotic stress.
- How Glycine Betaine Modifies Lipid Membrane Interactions,
Yuri Shakhman, Daniel Harries,
ChemSystemsChem 2021.
https://doi.org/10.1002/syst.202100010