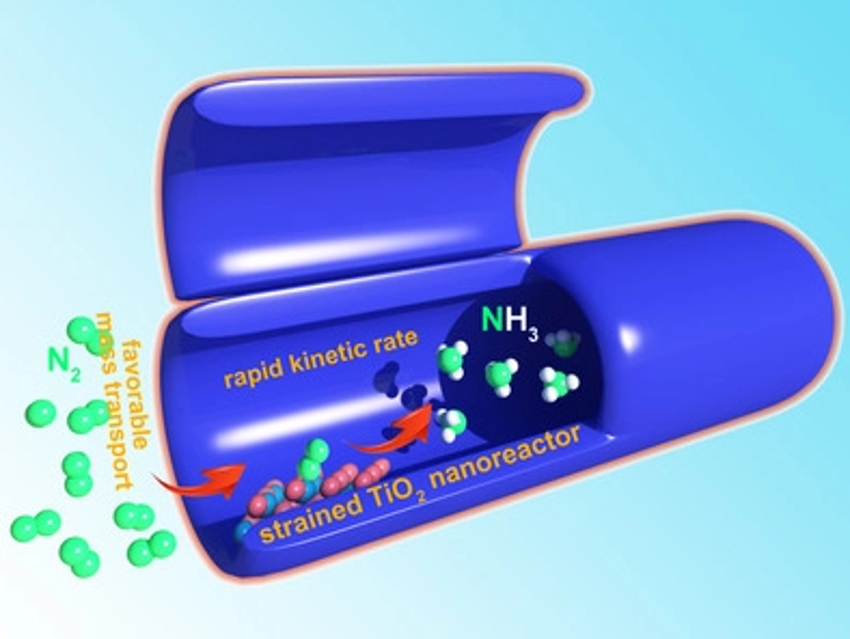
Electrochemical flow cells for the reduction of nitrogen and water to ammonia typically suffer from sluggish reaction kinetics and unfavorable mass transport phenomena. Due to these drawbacks, ammonia, which is a potential fuel with high hydrogen density, is often produced with poor selectivity and in low yields.
Guihua Yu, Zhaoyu Jin, and colleagues, University of Texas at Austin, USA, have developed a surface-strained TiO2 nanoreactor to enhance the electrocatalytic nitrogen reduction reaction. The nanotubular structure of the reactor regulates mass transport and offers an enlarged surface area. The team first prepared TiO2 nanotubes via a two-step anodization process and then converted them to lattice-strained TiO2 nanotubes via the electrochemical intercalation of lithium ions.
The team found that Ti3+ sites, which are stabilized by the lattice strain in the material, provide a more favorable pathway for nitrogen activation and allow for a faster kinetic rate than pristine TiO2. The nanoreactor delivers a high faradaic efficiency of 26 % and an ammonia yield rate of 16.67 µg h–1 mg–1. According to the researchers, the work could contribute to the development of optimized nanoreactors for the electrosynthesis of valuable products.
- A Surface-Strained and Geometry-Tailored Nanoreactor that Promotes Ammonia Electrosynthesis,
Panpan Li, Zhaoyu Jin, Zhiwei Fang, Guihua Yu,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202011596