β-Lactam antibiotics are a valuable class of drugs used to combat bacterial infections. Unfortunately, their overuse has led to the development of antibiotic resistances. These resistances are caused by the evolution of β-lactamases, a class of enzymes that can hydrolyze the β-lactam ring and render these antibiotics ineffective.
The enzyme New Delhi metallo-β-lactamase (NDM-1), for example, acts against nearly all β-lactam antibiotics. Thus, NDM-1 inhibitors such as aspergillomarasmine A (AMA, pictured below) are of interest. However, due to the complicated synthesis and derivatization of AMA, its development into a more potent and selective inhibitor is difficult.
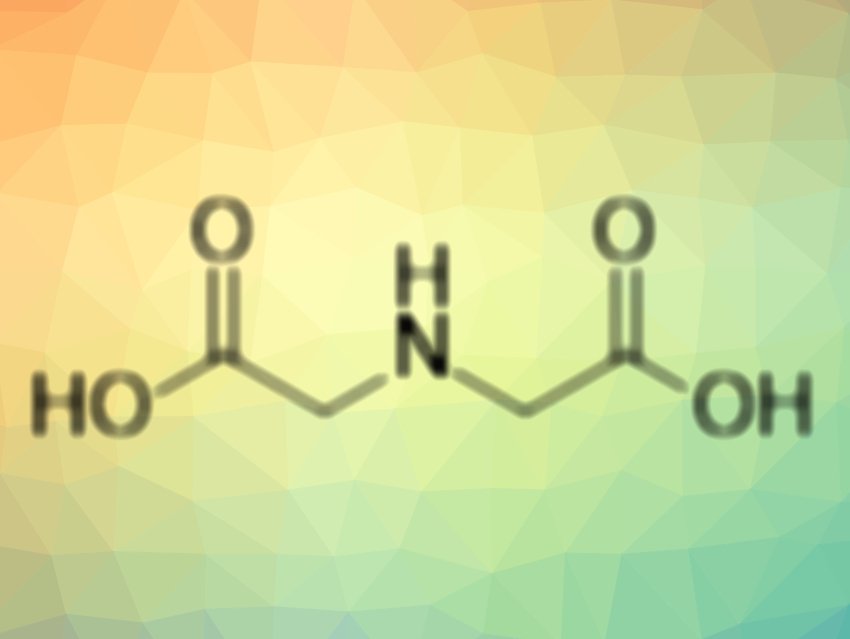
Seth M. Cohen, University of California, San Diego, USA, Michael W. Crowder, Miami University, Oxford, OH, USA, Walter Fast, University of Texas, Austin, USA, and colleagues have used iminodiacetic acid (IDA, pictured above), a metal-binding pharmacophore (MBP) core of AMA, for the fragment-based drug discovery of NDM-1 inhibitors. IDA is a simplified analogue of AMA and allows for easier derivatization. The team first performed a preliminary screening of the inhibition activity of IDA and structurally similar analogues. Then, the structure of the most promising compounds was further tuned to develop an effective inhibitor.
Native-state electrospray ionization mass spectrometry (ESI-MS) showed that the most promising inhibitors form stable ternary complexes with NDM-1, which inhibits the enzyme. Overall, the work could be helpful for the development of potent IDA-based inhibitors against metallo‐β‐lactamases, and thus, contribute to combating antibiotic resistance.
- Iminodiacetic Acid as a Novel Metal‐Binding Pharmacophore for New Delhi Metallo‐β‐lactamase Inhibitor Development,
Allie Y. Chen, Caitlyn A. Thomas, Pei W. Thomas, Kundi Yang, Zishuo Cheng, Walter Fast, Michael W. Crowder, Seth M. Cohen,
ChemMedChem 2020.
https://doi.org/10.1002/cmdc.202000123