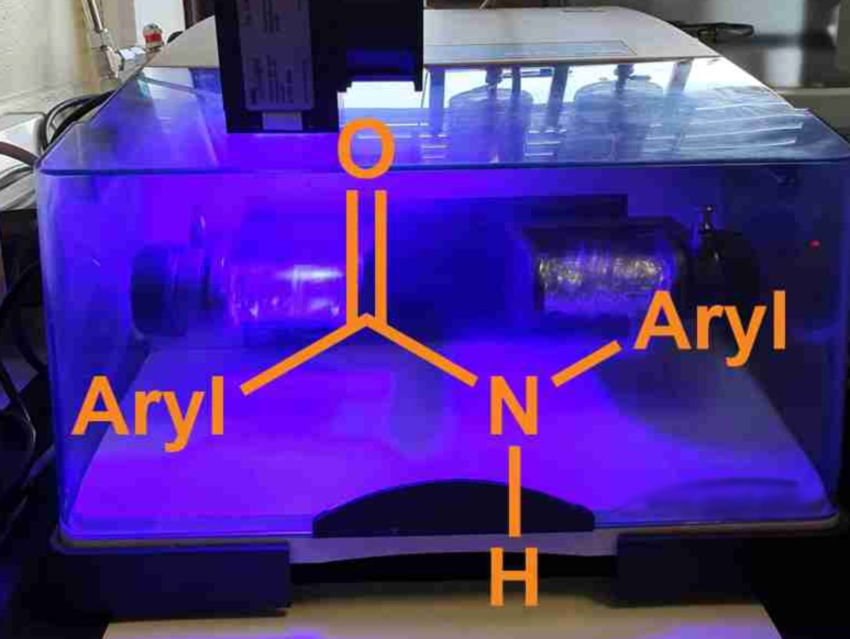
Amide groups are present in many pharmaceutical agents, agrochemicals, polymers, and natural products. The synthesis of aryl amides can be challenging when the amine reactants are poor nucleophiles. Many amidation approaches for this type of reaction, thus, require harsh reaction conditions, environmentally harmful solvents, high temperatures, and/or long reaction times. Developing “greener” processes with smaller solvent volumes, shorter reaction times, and reduced energy consumption would be useful.
Filipe Vilela, Heriot-Watt University, Edinburgh, UK, Gareth O. Lloyd, University of Lincoln, UK, and colleagues have developed two mechanochemical, solventless processes based on ball-milling for the synthesis of a variety of N-arylamides. The reactions achieve the N-amidation of O-protected hydroxamic acids using either aryl iodides or aryl boronic acids.
The variant using aryl iodides is mediated by stoichiometric amounts of copper(I) thiophene-2-carboxylate (CuTC) in the presence of N,N‘‐dimethylethylenediamine (DMEDA) using liquid-assisted grinding with small amounts of ethanol. The variant with aryl boronic acids uses copper(I) acetate, tBuOK as a base, and no liquid-assisted grinding component. The team used custom milling jars, which were made using a commercial 3D printer, and mixing balls with different diameters to allow the efficient mixing of the reactants.
Both protocols proved to be highly efficient and provided access to N-arylamides. Overall, the team achieved the synthesis of aryl amide compounds from protected hydroxamic acids in high yields (>90 %), in a scalable manner (up to multigram quantities), and with reduced reaction times (20 min). According to the team, this solventless synthesis may encourage other researchers to explore the possibilities of mechanochemistry.
- Mechanochemically Mediated Synthesis of N‐Aryl Amides from O‐protected Hydroxamic Acids,
Gareth Owen Lloyd, Emmanouil Broumidis, Mary C. Jones, Filipe Vilela,
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000451