Oligoheteroarenes, i.e., chains of heteroaromatic and aromatic molecules, are important scaffolds in the field of organic semiconductors. Oligothiophenes, for example, have applications in, e.g., organic photovoltaic materials, LEDs, fluorescence imaging, and other organic semiconducting materials. The synthesis of repetitive or symmetric molecules can be effectively carried out by polymerizations. Access to non-symmetric scaffolds, in contrast, involves a stepwise procedure that can be time-consuming and generate a lot of waste. However, non-symmetric structures can have benefits in terms of charge separation and material morphology.
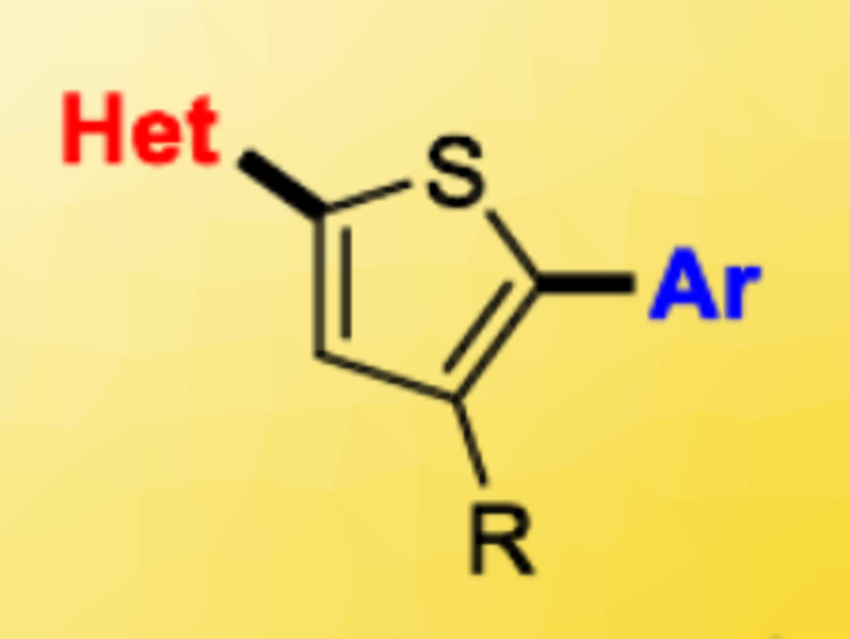
Pat Forgione, Concordia University, Montreal, Canada, and colleagues have developed a new route to access tri-aryl oligoheteroarenes. The team synthesized non-symmetric, di-aryl-substituted thiophenes (pictured above) using successive Pd‐catalyzed decarboxylative cross-coupling (DCC) reactions, with an intermediate saponification step (pictured below). They started from bromothiophene esters, which were coupled with heteroaromatic carboxylic acids in the presence of Pd(PtBu3)2 as the catalyst and KOH as a base. The ester group was then converted to a carboxylic acid by saponification, followed by a second DCC reaction to give the desired non-symmetric products. The researchers synthesized 16 different tri-aryls with overall yields ranging from 28–84 %.

This method is atom-economic and fast. The total reaction time is only 1.5 h, and the process requires just a single chromatography purification. This strategy allows the efficient synthesis of a library of semiconductor candidates, which can then be screened for desired properties.
- Successive Pd-Catalyzed Decarboxylative Cross-Couplings for the Modular Synthesis of Non-Symmetric Di-Aryl-Substituted Thiophene,
Cynthia Messina, Liam Douglas, Jiang Tian Liu, Pat Forgione,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000780