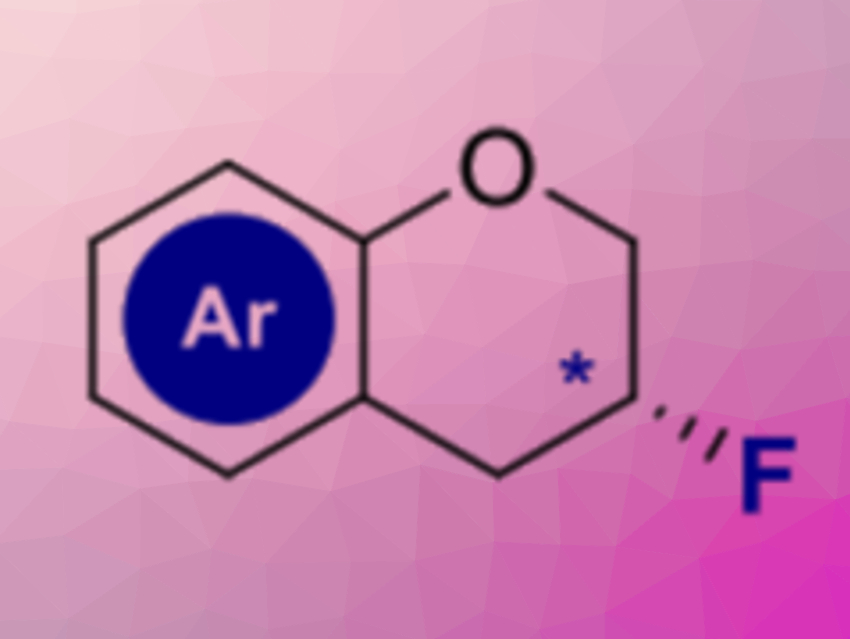
Changing the physicochemical properties of small molecules by fluorination is a useful strategy in drug discovery. Often, motifs that are commonly found in nature can be tuned for pharmaceutical applications by the strategic introduction of fluorine. The heterocycle chromane is found in numerous biologically active molecules, however, methods to access 3-fluorochromanes (pictured above) are rare.
Ryan Gilmour and colleagues, University of Münster, Germany, have developed a iodine-catalysis-based approach to convert allyl phenyl ethers into enantioenriched 3-fluorochromanes (pictured below). The team used a chiral, C2-symmetric iodoresorcinol-based catalyst, Selectfluor® as an oxidant, and amine/HF mixtures as reactants to create a variety of fluorinated chromanes in an enantioselective fashion.
A Selectfluor®‐mediated oxidation converts the catalyst to a hypervalent ArIF2 species in situ (pictured below). This species then enables the fluorocyclization of the substrate. The overall process is stereospecific with enantiomeric ratios up to 93:7 and allows rapid access to biologically relevant 3-fluorochromanes.

- Enantioselective Synthesis of 3‐Fluorochromanes via Iodine(I)/Iodine(III) Catalysis,
Jérôme C. Sarie, Christian Thiehoff, Jessica Neufeld, Constantin G. Daniliuc, Ryan Gilmour,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202005181