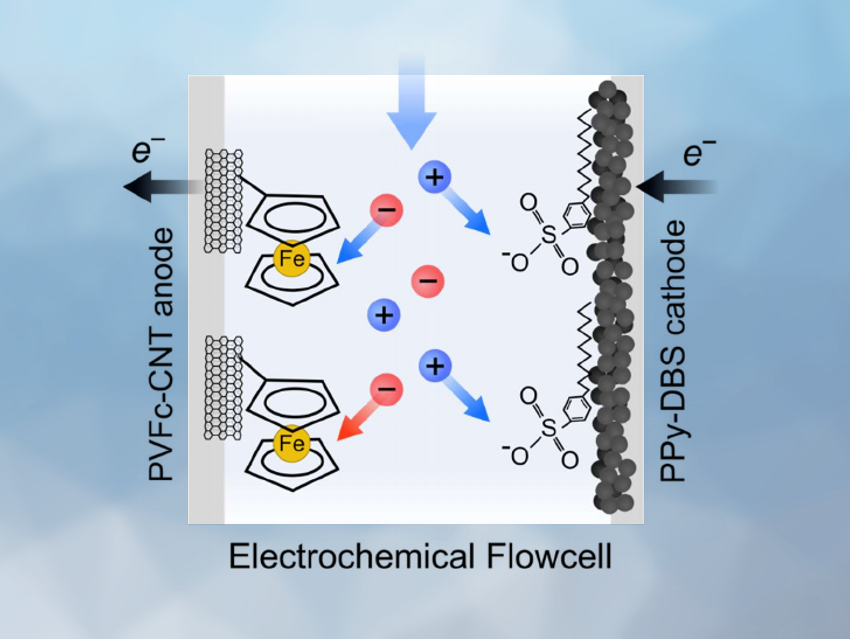
Limited access to clean water is a challenge in many regions of the world. This problem can be exacerbated by the industrial release of toxic heavy metal pollutants into the environment. Thus, effective methods for the removal and recovery of heavy metals—e.g., vanadium oxyanions from the smelting, ceramic, refinery, and battery industries—are important.
T. Alan Hatton, Massachusetts Institute of Technology (MIT), Cambridge, USA, and colleagues have developed an electrochemical flow platform for the reversible and selective recovery of vanadium(V) oxyanions from aqueous streams. The team harnessed the intrinsic affinity of the metallocene polymer polyvinylferrocene (PVFc) for oxyanion species. PVFc-functionalized carbon nanotubes (CNTs) were used as an anode (pictured above on the left) and the conducting polymer polypyrrole (PPy), doped with the anionic surfactant sodium dodecylbenzenesulfonate (SDBS), was used as the cathode (pictured above on the right) in an electrochemical flow cell. This setup was used for vanadium oxyanion removal from aqueous solutions in the presence of competing anions.
The anode adsorbs and sequesters vanadates during oxidation, while the cathode adsorbs and reduces the corresponding cations. When the redox reactions are reversed by switching the applied voltage, the adsorbed ions are removed from the electrodes and can be recovered. The team found that the flow cell has a high selectivity towards vanadium over competing anions. The method does not require the use of chemical agents to release the ions after sequestration, eliminating the waste problems associated with other separation approaches.
- Electrochemical selective recovery of heavy metal vanadium oxyanion from continuously flowing aqueous streams,
Ali Hemmatifar, Nil Ozbek, Cameron Halliday, T. Alan Hatton,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202001094