Supercritical carbon dioxide (scCO2) is a promising reaction medium that satisfies green chemistry principles such as renewability and low toxicity. Supercritical carbon dioxide can, for example, be useful in the controlled and environmentally benign chemical synthesis of metal–organic frameworks (MOFs)—porous materials with a wide range of applications. The exact relationship between synthetic parameters and synthesis outcomes, however, is often difficult to predict for this type of reaction.
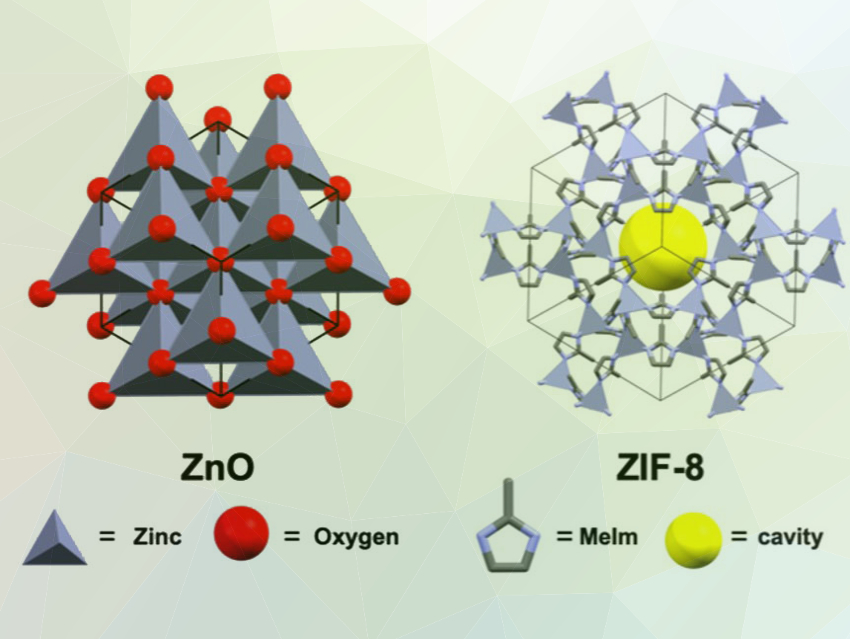
Michael A. Sinnwell, Praveen K. Thallapally, Pacific Northwest National Laboratory (PNNL), Richland, WA, USA, and colleagues have monitored the synthesis of the commercially used MOF ZIF-8 (pictured right, MeIm = 2‐methylimidazate) in scCO2 in real time. The MOF was synthesized from ZnO templates by reacting them with HMeIm. The team used in situ X-ray diffraction and scanning electron microscopy (SEM) to systematically study nucleation and growth processes during the synthesis. They evaluated the impact of the structure of the templates, the reaction time, the density of the supercritical solvent, and the temperature on the product composition, size, and morphology.
The team found that factors such as the size and shape of the sacrificial ZnO templates can influence the properties of the product. The rate of ZIF‐8 formation depends on the surface area of the templating ZnO source. These insights could be used to control the morphology of ZIF-8 and ZIF-8 composites and provide a path towards tailored materials.
- Kinetics and mechanisms of ZnO to ZIF-8 transformations in supercritical CO2 revealed by in situ X-ray diffraction,
Michael Sinnwell, Quin R. S. Miller, Lili Liu, Jinhui Tao, Mark E. Bowden, Libor Kovarik, Dushyant Barpaga, Yi Han, Radha Kishan Motkuri, Maria L. Sushko, Herbert T. Schaef, Praveen K. Thallapally,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202000434
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

