New Approach to Selective Chemotherapy
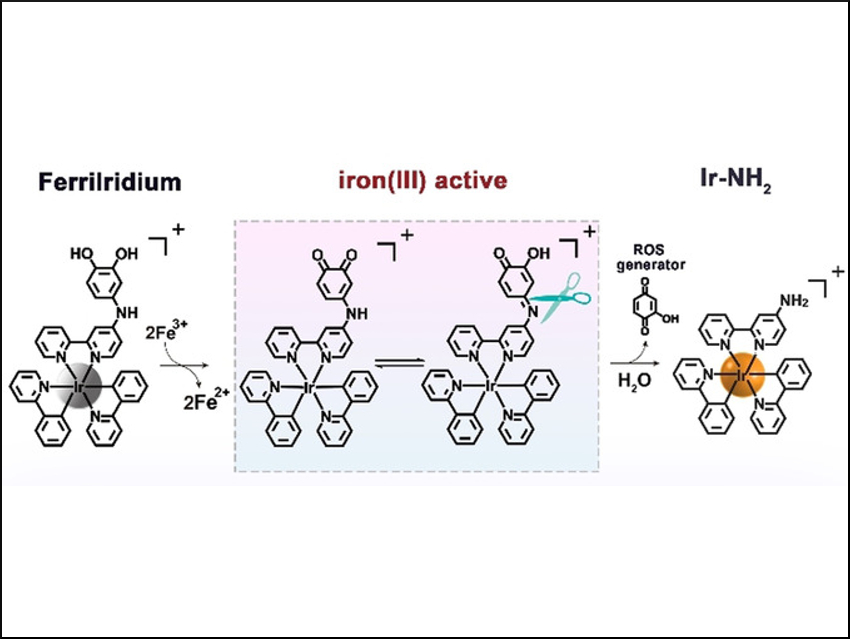
A new drug, named “FerriIridium”, can simultaneously help diagnose and treat gastric cancer. The initially weakly active precursor (prodrug), based on an iridium-containing compound, is selectively activated only after reaching the interior of a tumor cell. This is possible because of the higher amount of iron present there. This selective activation reduces undesired side effects.
Cells transport substances from their exterior to their interior by folding in small regions of their membrane and then binding them off (endocytosis). This is how FerriIridium enters target cells. The resulting vesicles then fuse with lysosomes. These cell organelles have an acidic environment that contains trivalent iron ions, Fe(III), and enzymes, with which they dismantle cell components that are no longer needed. In gastric cancer cells, the Fe(III) concentration within the lysosomes is significantly elevated.
Yu Chen, Sun Yat-Sen University, Guangzhou, China, Hui Chao, Sun Yat-Sen University and Hunan University of Science and Technology, Xiangtan, China, and colleagues have made use of this feature. They equipped FerriIridium with a special functional group (the m-iminocatechol group) that selectively binds to Fe(III). When bound, the functional group is oxidized while the iron ions are reduced to Fe(II). Under the acidic conditions within the lysosomes, the FerriIridium is then split into two components: an iridium complex and a benzoquinone derivative.
Triple Effect
This reaction mechanism has a threefold effect. First, Fe(II) ions can catalyze a reaction that produces highly reactive hydroxyl radicals. Second, benzoquinones are highly oxidizing. With certain cellular substances, such as NADPH, they form hydroxyquinones, which react with oxygen to produce radical oxygen species, as well as hydrogen peroxide. These species, in turn, can react with Fe(II) to produce hydroxyl radicals. Benzoquinone compounds can also disrupt cellular respiration. The radicals destroy the lysosomes, releasing their contents. Third, the splitting of FerriIridium drastically increases both the phosphorescence and the toxicity of the iridium complex. The phosphorescence can be used to diagnose the tumor.
Most importantly, however, the toxic iridium complex is absorbed by mitochondria, the “cellular power plants”. It destroys them from the inside out by collapsing their membrane potential. Together, these effects lead to the death of the gastric cancer cells and shrinking of the tumors, as demonstrated by experiments on cell lines and mice.
- FerriIridium: A Lysosome-Targeting Iron(III)-Activated Iridium(III) Prodrug for Chemotherapy in Gastric Cancer Cells,
Shi Kuang, Xinxing Liao, Xianrui Zhang, Thomas W. Rees, Ruilin Guan, Kai Xiong, Yu Chen, Liangnian Ji, Hui Chao,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201915828