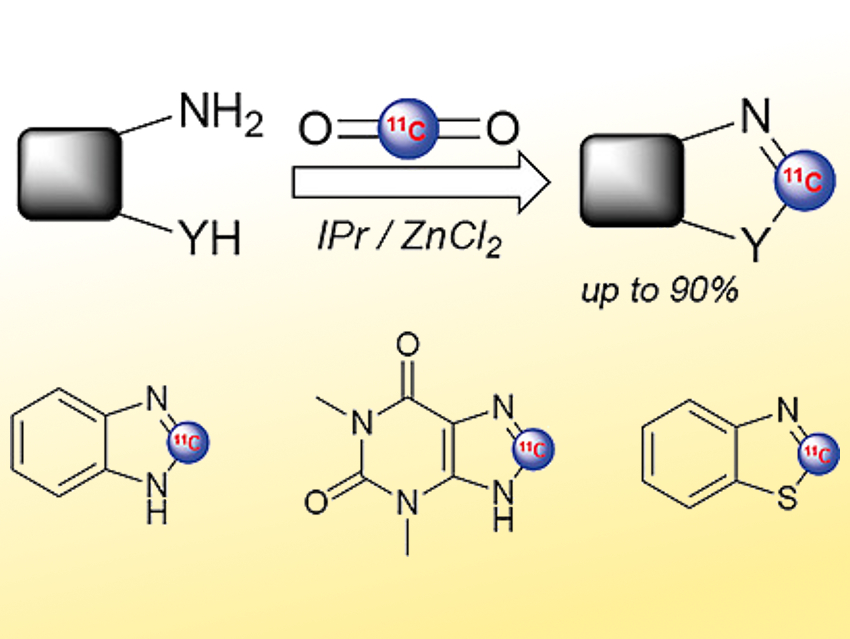
Positron emission tomography (PET) is a medical imaging technique used in diagnostics and drug development. This method requires the labeling of molecules with positron‐emitting radioisotopes, such as carbon-11 or fluorine-18. Carbon-11 makes it simple to obtain a radiolabeled equivalent of existing drugs by isotopic replacement of a carbon atom. However, its short half-life of only 20 minutes requires rapid and efficient reactions. Carbon-11 is produced by a cyclotron, mainly as [11C]CO2. Generally, this CO2 is first transformed into the more reactive species [11C]CH3I or [11C]CH3OTf by multistep processes and then used to perform methylation reactions at peripheral positions of molecules.
Thierry Billard, CERMEP In-Vivo Imaging Center, Lyon, France, and University of Lyon, Villeurbanne, France, and colleagues have developed a way to directly use cyclotron-produced [11C]CO2 to synthesize heterocyclic molecules labeled intracyclically (pictured). The team converted, e.g., aromatic diamines to benzimidazoles or aminobenzenethiols to benzothiazoles. They used Ph2SiH2 as a reducing agent, the N-heterocyclic carbene (NHC) 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene as a ligand, ZnCl2 as a catalyst, and diglyme (bis(2-methoxyethyl) ether) as the solvent.
The labeling can be performed in only ten minutes, which is compatible with the short carbon-11 half-life, and with good radiochemical yields. Using this method, the team obtained various radiolabeled heterocyclic structures (examples pictured). For instance, an 11C-radiolabeled variant of the drug theophylline (pictured bottom center) has been synthesized.
- 11C-Labeling: Intracyclic Incorporation of Carbon-11 into Heterocycles,
François Liger, Florence Cadarossanesaib, Thibaut Iecker, Christian Tourvieille, Didier Le Bars, Thierry Billard,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901386